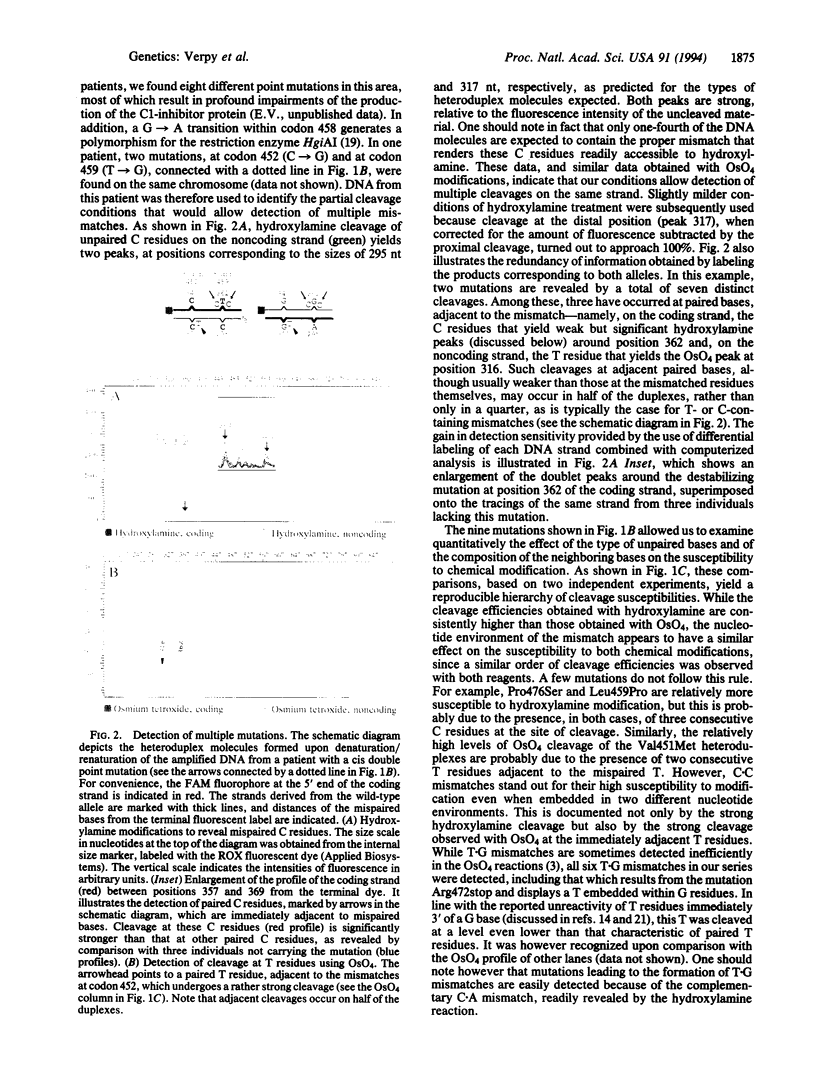
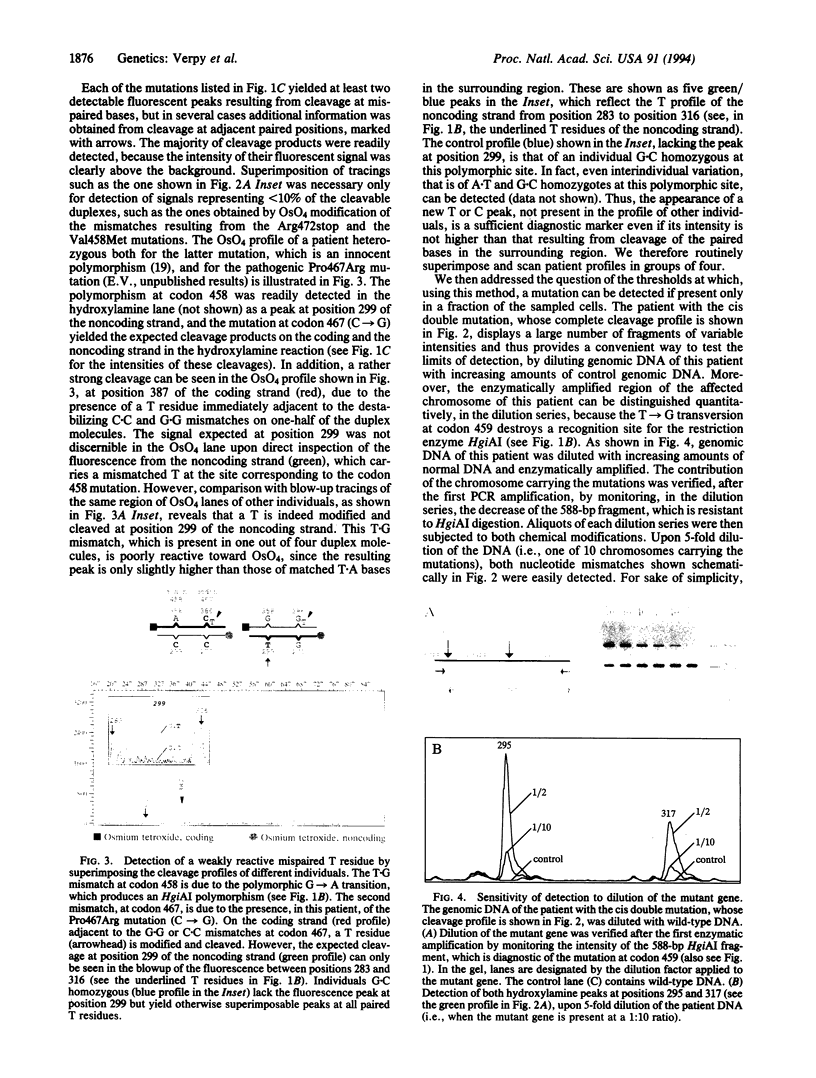
Abstract
Presently available methods for screening large genetic regions for unknown point mutations are neither flawless nor particularly efficient. We describe an approach, especially well suited to identifying mutations present in the heterozygous state, that combines several improvements in a protocol called fluorescence-assisted mismatch analysis (FAMA). Appropriate gene regions of the wild-type and the putative mutant allele are simultaneously amplified from genomic DNA by using the polymerase chain reaction, and large DNA fragments, so far up to 800 bp, are end labeled with strand-specific fluorophores. Aliquots are denatured and reannealed to form heteroduplexes and subjected to conventional cytosine- and thymine-specific modifications. Cleavages occurring on opposite strands are detected by denaturing gel electrophoresis using an automated DNA sequencer. Since the DNA fragments derived from the mutant allele are also end labeled, the number of informative mispaired bases is doubled compared to conventional searches using wild-type probes. The sensitivity of detection is also increased, because differential fluorescent end labelling allows the identification and measurement of strand-specific background cleavages at matched cytosine or thymine residues. Automatic superimposition of tracings from different subjects allows mismatch detection at sites that, because of the nature of the bases involved and of the neighboring sequence, are known to be less susceptible to cleavage. The effects of the latter parameters have been studied quantitatively on a series of point mutations found in the human C1-inhibitor gene in patients affected by hereditary angioedema. Dilution experiments have demonstrated that most mutations are detected even when the mutant chromosome is diluted 10-fold or more compared with the normal one.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. J., Milner C. M., Cotton R. G., Campbell R. D. The coding sequence of the hemolytically inactive C4A6 allotype of human complement component C4 reveals that a single arginine to tryptophan substitution at beta-chain residue 458 is the likely cause of the defect. J Immunol. 1992 May 1;148(9):2795–2802. [PubMed] [Google Scholar]
- Bock S. C., Skriver K., Nielsen E., Thøgersen H. C., Wiman B., Donaldson V. H., Eddy R. L., Marrinan J., Radziejewska E., Huber R. Human C1 inhibitor: primary structure, cDNA cloning, and chromosomal localization. Biochemistry. 1986 Jul 29;25(15):4292–4301. doi: 10.1021/bi00363a018. [DOI] [PubMed] [Google Scholar]
- Carter P. E., Duponchel C., Tosi M., Fothergill J. E. Complete nucleotide sequence of the gene for human C1 inhibitor with an unusually high density of Alu elements. Eur J Biochem. 1991 Apr 23;197(2):301–308. doi: 10.1111/j.1432-1033.1991.tb15911.x. [DOI] [PubMed] [Google Scholar]
- Costes B., Girodon E., Ghanem N., Chassignol M., Thuong N. T., Dupret D., Goossens M. Psoralen-modified oligonucleotide primers improve detection of mutations by denaturing gradient gel electrophoresis and provide an alternative to GC-clamping. Hum Mol Genet. 1993 Apr;2(4):393–397. doi: 10.1093/hmg/2.4.393. [DOI] [PubMed] [Google Scholar]
- Cotton R. G. Current methods of mutation detection. Mutat Res. 1993 Jan;285(1):125–144. doi: 10.1016/0027-5107(93)90060-s. [DOI] [PubMed] [Google Scholar]
- Cotton R. G., Rodrigues N. R., Campbell R. D. Reactivity of cytosine and thymine in single-base-pair mismatches with hydroxylamine and osmium tetroxide and its application to the study of mutations. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4397–4401. doi: 10.1073/pnas.85.12.4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dianzani I., Forrest S. M., Camaschella C., Gottardi E., Cotton R. G. Heterozygotes and homozygotes: discrimination by chemical cleavage of mismatch. Am J Hum Genet. 1991 Feb;48(2):423–424. [PMC free article] [PubMed] [Google Scholar]
- Fischer S. G., Lerman L. S. DNA fragments differing by single base-pair substitutions are separated in denaturing gradient gels: correspondence with melting theory. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1579–1583. doi: 10.1073/pnas.80.6.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest S. M., Dahl H. H., Howells D. W., Dianzani I., Cotton R. G. Mutation detection in phenylketonuria by using chemical cleavage of mismatch: importance of using probes from both normal and patient samples. Am J Hum Genet. 1991 Jul;49(1):175–183. [PMC free article] [PubMed] [Google Scholar]
- Grompe M., Caskey C. T., Fenwick R. G. Improved molecular diagnostics for ornithine transcarbamylase deficiency. Am J Hum Genet. 1991 Feb;48(2):212–222. [PMC free article] [PubMed] [Google Scholar]
- Grompe M., Gibbs R. A., Chamberlain J. S., Caskey C. T. Detection of new mutation disease in man and mouse. Mol Biol Med. 1989 Dec;6(6):511–521. [PubMed] [Google Scholar]
- Grompe M., Muzny D. M., Caskey C. T. Scanning detection of mutations in human ornithine transcarbamoylase by chemical mismatch cleavage. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5888–5892. doi: 10.1073/pnas.86.15.5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu A. L., Hsu I. C. Detection of single DNA base mutations with mismatch repair enzymes. Genomics. 1992 Oct;14(2):249–255. doi: 10.1016/s0888-7543(05)80213-7. [DOI] [PubMed] [Google Scholar]
- Montandon A. J., Green P. M., Giannelli F., Bentley D. R. Direct detection of point mutations by mismatch analysis: application to haemophilia B. Nucleic Acids Res. 1989 May 11;17(9):3347–3358. doi: 10.1093/nar/17.9.3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orita M., Iwahana H., Kanazawa H., Hayashi K., Sekiya T. Detection of polymorphisms of human DNA by gel electrophoresis as single-strand conformation polymorphisms. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2766–2770. doi: 10.1073/pnas.86.8.2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. G., Bobrow M., Bentley D. R. Point mutations in the dystrophin gene. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2331–2335. doi: 10.1073/pnas.89.6.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues N. R., Rowan A., Smith M. E., Kerr I. B., Bodmer W. F., Gannon J. V., Lane D. P. p53 mutations in colorectal cancer. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7555–7559. doi: 10.1073/pnas.87.19.7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar G., Yoon H. S., Sommer S. S. Screening for mutations by RNA single-strand conformation polymorphism (rSSCP): comparison with DNA-SSCP. Nucleic Acids Res. 1992 Feb 25;20(4):871–878. doi: 10.1093/nar/20.4.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield V. C., Beck J. S., Kwitek A. E., Sandstrom D. W., Stone E. M. The sensitivity of single-strand conformation polymorphism analysis for the detection of single base substitutions. Genomics. 1993 May;16(2):325–332. doi: 10.1006/geno.1993.1193. [DOI] [PubMed] [Google Scholar]
- Sheffield V. C., Cox D. R., Lerman L. S., Myers R. M. Attachment of a 40-base-pair G + C-rich sequence (GC-clamp) to genomic DNA fragments by the polymerase chain reaction results in improved detection of single-base changes. Proc Natl Acad Sci U S A. 1989 Jan;86(1):232–236. doi: 10.1073/pnas.86.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smooker P. M., Cotton R. G. The use of chemical reagents in the detection of DNA mutations. Mutat Res. 1993 Jul;288(1):65–77. doi: 10.1016/0027-5107(93)90208-w. [DOI] [PubMed] [Google Scholar]
- Stoppa-Lyonnet D., Duponchel C., Meo T., Laurent J., Carter P. E., Arala-Chaves M., Cohen J. H., Dewald G., Goetz J., Hauptmann G. Recombinational biases in the rearranged C1-inhibitor genes of hereditary angioedema patients. Am J Hum Genet. 1991 Nov;49(5):1055–1062. [PMC free article] [PubMed] [Google Scholar]
- Stoppa-Lyonnet D., Tosi M., Laurent J., Sobel A., Lagrue G., Meo T. Altered C1 inhibitor genes in type I hereditary angioedema. N Engl J Med. 1987 Jul 2;317(1):1–6. doi: 10.1056/NEJM198707023170101. [DOI] [PubMed] [Google Scholar]