Introduction
The utility of the pericardial space in the electrophysiology lab for mapping and ablation was not appreciated by cardiac electrophysiologists until percutaneous access to the epicardial space for catheter ablation of ventricular tachycardia (VT) was first described by Sosa and colleagues in patients with Chagasic cardiomyopathy in 1997.1 Long regarded as an area accessed only if procedural complications developed, the epicardial space has now become the new frontier in expanding the role of cardiac catheter ablation and therapeutics.2, 3 For many years patients with arrhythmias (which could not be ablated endocardially) were treated with an open surgical approach, reminiscent of the first bypass tract surgical resection in a Wolff-Parkinson-White (WPW) patient in 1969.4, 5
The focus of this paper is to review the development of epicardial interventions in electrophysiology in the last decade and a half, and to provide an update on the current status and future prospects in this field. The anatomy of the epicardial space is discussed initially, as this provides a basis for a review of the relevant imaging aspects. The technique of accessing the space, initially developed for drainage of pericardial effusions by pericardiocentesis and subsequently modified for accessing a normal pericardial space, will also be outlined. We will also discuss mapping and ablation of arrhythmias from the epicardial surface of the heart. The majority of epicardial clinical studies have involved ventricular arrhythmias, with only small series or case reports for supraventricular arrhythmias.
The most recent EHRA/HRS consensus document reported that the epicardial space was accessed in 17% of VT ablation procedures, based on a survey of VT tertiary referral centers.6 A recent multicenter study from tertiary referral centers found an overall epicardial access rate of 19% for patient undergoing VT ablation procedures.7 This ranged from 6% in ‘normal’ hearts, to 16% for ischemic cardiomyopathy (CM), 35% for dilated CM and 41% for arrhythmogenic right ventricular cardiomyopathy (ARVC). However, as the technique becomes more widely available, these numbers are likely to increase.
Anatomy: (Fig 18, 2A& B9, Videos 1–8, supplemental figure 1)
Figure 1.
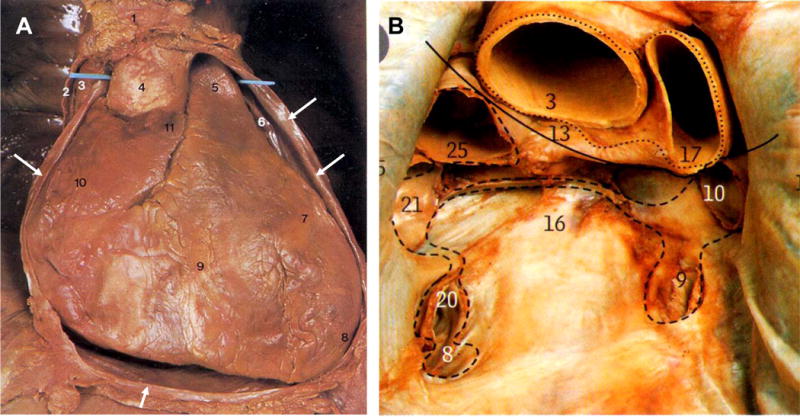
Pericardial Anatomy. Panel A: View of the heart with the anterior chest wall removed and the parietal pericardium cut-open (2, and arrows point to cut edges). The blue probe runs in the transverse sinus between the aorta (4) and PA (5), the right atrial appendage (11), left atrial appendage (6), the right ventricle (9), left ventricle (7), superior vena cava (3). Panel B: view of pericardial cavity after removal of the heart, the dotted lines show cut edges of the pericardium showing the oblique sinus (16) and marker in transverse sinus (13), ascending aorta (3), pulmonary artery (17), superior van cava (25), left superior pulmonary vein (10), left inferior pulmonary vein (9), right superior pulmonary vein (21), right inferior pulmonary vein (20), inferior vena cava (8). (figure 1 used with permission: McMinn’s Clinical Anatomy: Mosby-Elsevier8)
Figure 2.
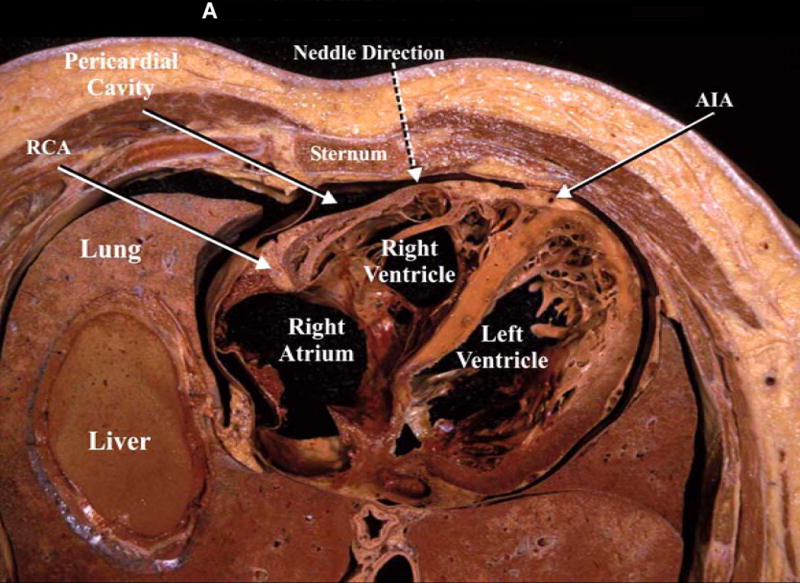
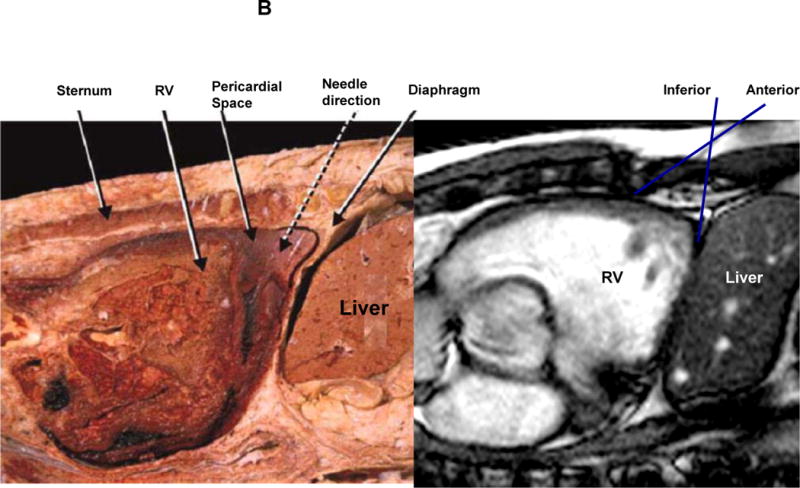
A: The figure demonstrates a cross section of the lower part of the thorax revealing the relationships of the pericardial cavity with the adjacent structures. In addition, the broken arrow is illustrating the direction of the pericardiocentesis needle. RCA, right coronary artery; AIA, anterior interventricular artery. B: midsagittal section of a cadaver revealing the pericardial cavity, the diaphragm and the contents of the abdominal cavity. In addition, the broken arrow shows the direction of the pericardiocentesis needle [fig 2 A and 2B (left panel) used with permission from Lukas et al9]
With the development of the percutaneous epicardial mapping and ablation approach, knowledge of the pericardial space anatomy is important for cardiac electrophysiologists using this approach for catheter ablation of arrhythmias.10–12 The heart is located within a double layered membrane known as the pericardium extending from the roots of the great vessels to the central tendon of the diaphragm. It consists of an outer fibrous layer and an inner serous sac which is invaginated by the heart. The serous pericardium has a visceral layer on the epicardial surface and a parietal layer reflected on the outer fibrous layer. The thickness of the parietal pericardium varies from 0.8–2.5mm. The pericardial space is a potential space between the visceral and parietal layers of the pericardium, and normally contains <50mL of serous fluid. The reflection of the visceral pericardium at the posterior surface of the heart results in an oblique sinus bounded by the inferior vena cava (IVC) and the four pulmonary veins and situated posterior to the left atrium and anterior to the esophagus. It is the most posterior pericardial space, and is inferior to the transverse sinus from which it is separated by the pericardial reflections. The transverse sinus is located posterior to the ascending aorta and the pulmonary trunk and above the left atrium. As the pericardial reflections are located posteriorly, the anterior, apical and lateral surfaces of the ventricles are thus freely accessible within the pericardial space. In addition the posterior and superior surfaces of the left atrium are also accessible.
Nerve Supply
The fibrous pericardium, and the parietal layer of serous pericardium that lines it, are supplied by the phrenic nerves (located on the anterior lateral pericardial surface) and receives vagal inputs from the esophageal plexus.13 The visceral pericardial layer on the cardiac surface is insensitive. The pain of pericarditis arises in the parietal layer only, and is transmitted by the phrenic nerve. The phrenic nerves course along the parietal pericardium and their anatomic location needs to be understood as it is crucial to protect them during both endocardial and epicardial procedures.
Pericardial Anomalies
Congenital defects of the pericardium include both partial and rarely, total absence of the pericardium is reported in 1:10,000 autopsies, and may be associated with other cardiac anomalies. In approximately 75% of cases the pericardium is completely absent, and the heart and lungs occupy a common serous cavity; in the remainder of cases there is a partial defect, with a foramen of variable size connecting the pericardial and pleural cavities.14
Pericardial Imaging (fig 3 A&B, Videos 1–8)
Figure 3.
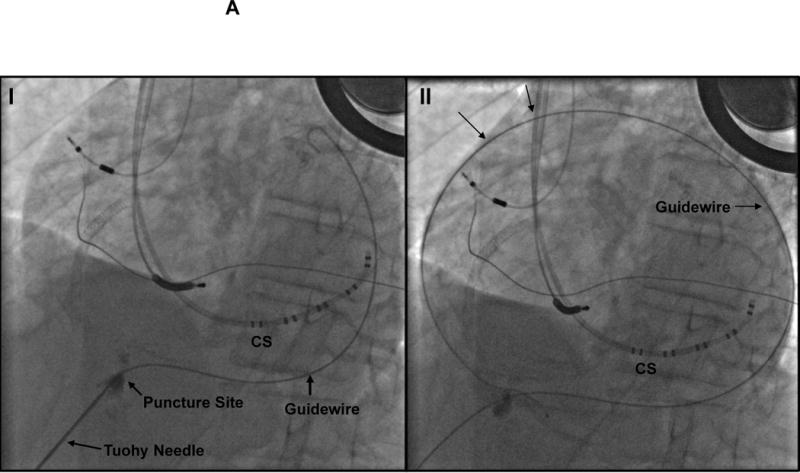
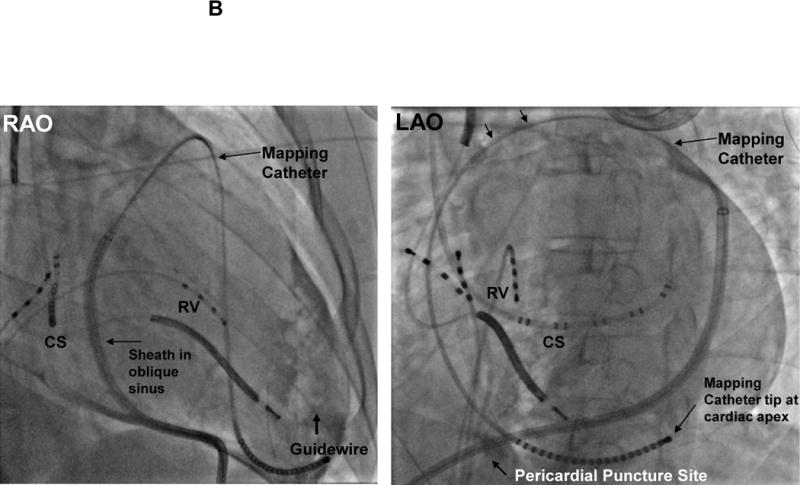
A: Pericardial Access Fluoroscopic Images: Panel I Puncture site of the pericardium (is in an inferior location) and advancement of the wire in the LAO (left anterior oblique view) is shown. CS= coronary sinus catheter, Panel II: the wire is now coursing around the heart B: Pericardial Mapping Fluoroscopic images: Left panel; right anterior oblique (RAO) view. Sheath in pericardial space and a multielectrode mapping catheter coursing in front of the aorta and the pulmonary trunk and descending down the RV free wall and reaching the apex of the ventricles. Right Panel; LAO view CS= coronary sinus catheter, RV= right ventricular catheter, RAO= right anterior oblique view, LAO= left anterior oblique view.
When contrast is introduced into the pericardial space, it flows freely and outlines the cardiac borders, sinuses and the pericardial reflections at the roots of the great vessels. [Online videos 4 and 7] CT and MR imaging can provide information on the pericardial space, specifically the pericardial recesses, pericardial abnormalities and a wide variety of systemic conditions affecting the pericardial space.15 Knowledge of the pericardial recesses, which arise from the sinuses, is important for correct interpretation of pericardial imaging.16, 17 The superior and inferior aortic recesses arise from the transverse sinus, the posterior cardiac recess arises from the oblique sinus and the pulmonary vein recesses and the post caval recess arise from the main pericardial cavity.
Accessing the Pericardial Space
The normal pericardial space contains 15–50 ml of serous fluid. While this provides for free movement between the visceral and parietal pericardial surfaces, it also provides the key to accessing the space in patients without pathologic pericardial effusions. The subxiphoid approach is the best known and most frequently used for percutaneous access; other approaches such as parasternal or apical can also be used.9 Additionally, transesophogeal,18 transatrial19, 20 and transbronchial21 methods to access the pericardial space have also been described.
Subxiphoid Approach (Fig 2 A&B, Fig 3 A&B, Fig 4 and Videos 1–8, supplemental figure 1)
Figure 4.
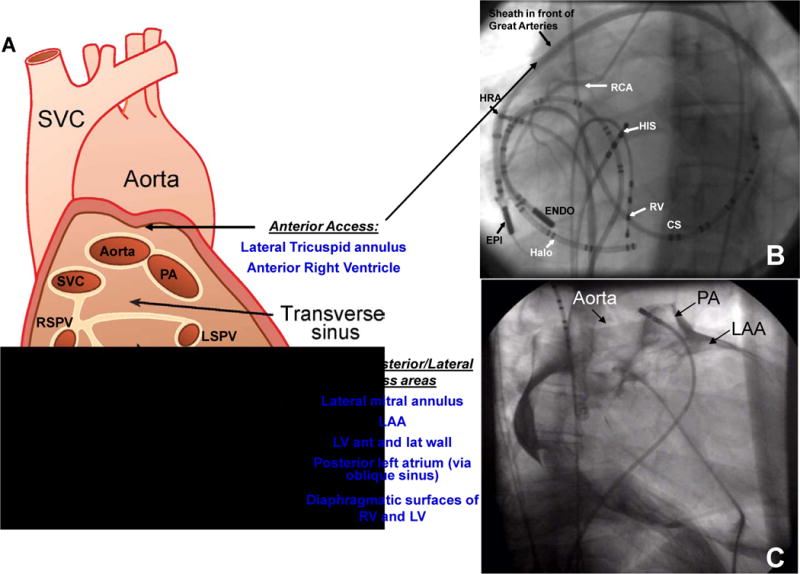
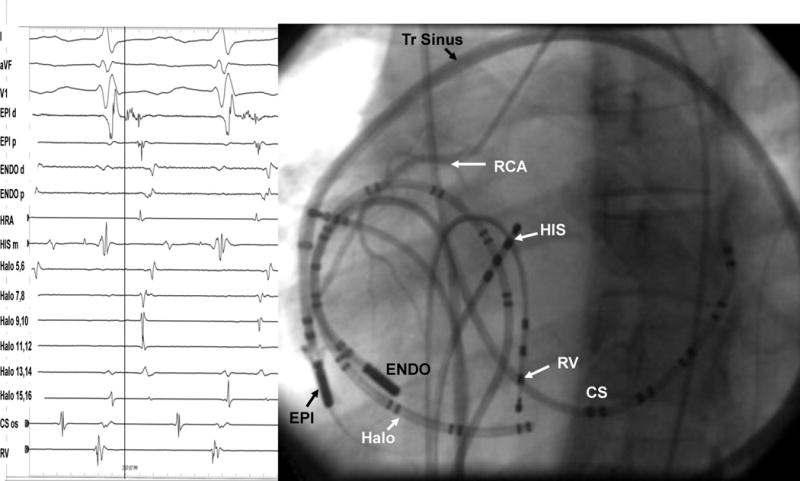
Pericardial sinuses and Fluoroscopic Views. Panel A: Cut edges of the pericardium demonstrating the pericardial sinuses. SVC=superior vena cava, PA= pulmonary artery, LSPV= left superior pulmonary vein, LIPV= left inferior pulmonary vein, RSPV=right superior pulmonary vein, RIPV=right inferior pulmonary vein, LAA=left atrial appendage, RV= right ventricle, LV=left ventricle. Panel B: Left anterior oblique fluoroscopic view of the catheters. CS = coronary sinus catheter; ENDO = endocardial ablation catheter; EPI = epicardial ablation catheter through a long sheath; Halo=tricuspid annulus catheter; HIS = His-bundle catheter; HRA =high right atrium; RCA =right coronary artery; RV = right ventricular catheter. Panel C: Contrast imaging of the pericardium showing the oblique sinus and other structures. LAA=left atrial appendage, PA=pulmonary artery. The pigtail catheter is deep in the oblique sinus.
In the standard subxiphoid approach, the skin in the subxiphoid region is sterilized and anesthetized with lidocaine 1%.2 If the procedure is performed under general anesthesia, then lidocaine 1% may be used for local anesthesia prior to removal of the sheath. A 17 G standard Tuohy needle (marketed by multiple manufacturers, originally designed for epidural access: Havel’s Inc, Cincinnati, OH; BD Medical, Franklin lakes, NJ) is used; either 3 ½ or 6 inch in length is commonly used (See appendix/figure 1). With the needle directed to the left shoulder, the skin is punctured between the left border of the subxiphoid process and the left rib. The curved part of the needle is the generally directed toward the heart. The angle between the needle and the thorax determines the area of the ventricle accessed; with a steeper angle (with respect to the chest wall), the access tends to be more posterior, while a less acute angle will approach the anterior surface. As the needle is advanced, the area between the diaphragm and the chest wall is crossed and as the fibrous pericardium is indented, cardiac pulsation may be appreciated. Injection of small amounts of contrast will indicate the location of the needle tip (Video 1), and once the fibrous pericardium is tented, this can be outlined with contrast (Video 2). Care should be taken at this step to avoid a static needle position especially if there is downward movement of the diaphragm. If the needle is not moved backward to compensate for the extent the diaphragm moves down, the ’net’ movement of the needle would be toward the heart (hence risking perforation of the heart). If the patient is under general anesthesia control of respiration at the time of pericardial puncture is helpful. Puncture of the fibrous pericardium is appreciated with a release of resistance on the needle and injection of contrast at this time will result in a contrast layer outlining the heart in the pericardial space. A guide wire is then advanced into the pericardial space and in the left anterior oblique (LAO) view it is observed to follow the left cardiac border, and preferably cross from the left to right side in front of the great vessels, confirming the pericardial location. In our lab, a 4F sheath is then advanced over the guide wire, and 5–10cc of contrast is injected into the pericardial space, which will outline the cardiac borders, sinuses and the roots of the great vessels (see videos 2–8). The presence of adhesions (video 10) can also be appreciated at this stage (adhesions may be present even in patients who have not had a previous thoracotomy). A long exchange wire is then introduced into the space over which, in our laboratory, an 8F SL0 sheath (St Jude Medical, Minnetonka, MN) is then advanced into the space, through which a mapping or ablation catheter can then be introduced. In the normal pericardial space, the catheter will move smoothly over the epicardial surface, allowing for easy mapping and ablation. Deflectable sheaths are also available for use in the pericardial space (Agilis EPI™, St. Jude Medical, Minnetonka, MN)
Surgical Approach
In patients who have pericardial adhesions, most commonly after prior cardiac surgical procedures, a limited thoracotomy approach can be used to access the pericardial space. Soejima and colleagues first described using a subxiphoid surgical approach with manual lysis of adhesions in six patients.22 Access to the diaphragmatic surface of the heart was achieved in all patients, with catheter manipulation to the lateral and anterior wall possible in four patients. Our group has reported on the use of surgical access in a series of 14 patients with prior unsuccessful endocardial ablation.23 The subxiphoid approach was used in 11 patients and 3 patients had a limited anterior thoracotomy to access the epicardium. The indications for surgical access was prior cardiac surgery (n=12), previous failed epicardial access (n=1) and need for ablation at a site in close proximity to phrenic nerve and coronary artery (n=1). While mapping was limited to the inferior and parts of posterior and lateral walls with the subxiphoid approach, the limited anterior thoracotomy provided access to the apex, anterior and anterolateral walls. Surgical access with limited thoracotomy proved feasible and safe in the cardiac EP lab, with the surgical approach tailored to the region of the ventricle to be mapped and ablated. Sub-xiphoid needle puncture is possible after cardiac surgery as proposed by Sosa et al24. It certainly requires more experience and is limited by the risk of multiple right ventricular (RV) punctures and a possible increase in the risk of RV pseudoaneurysm.
Epicardial Ventricular Tachycardia (Fig 5&6, video 9, Table I)
Figure 5.
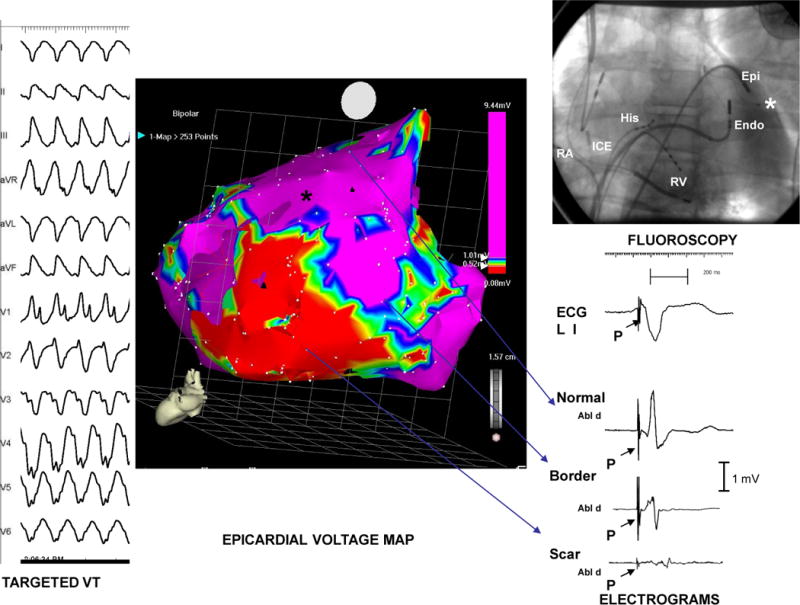
Epicardial Map: Non-Ischemic Cardiomyopathy. The far left panel of this picture displays the 12-lead ECG of a monomorphic VT induced in a patient with idiopathic dilated (non-ischemic) cardiomyopathy. The posterior view of the epicardial voltage map, generated during bi-V pacing, from the same patient is displayed in the center panel. The upper right panel displays a single fluoroscopic image in the LAO view with intracardiac catheters labeled; ablation catheters have been positioned near the border-zone of the scar in both the endocardium and epicardium. Finally, the right lower panel displays electrograms obtained from normal epicardial muscle, epicardial scar border zone, and regions of epicardial scar. Note the progressive widening of electrogram duration, reduction in slew rate and decreased amplitude between normal muscle to scar. Abbreviations: RA- right atrium; ICE- Intra-cardiac echo; RV- right ventricle; Endo- endocardium; Epi- epicardium, Abl d=Ablation distal recording * Denotes location of LV pacing lead of the resynchronization device. P=pacing spike (modified from Cesario et al29)
Figure 6.
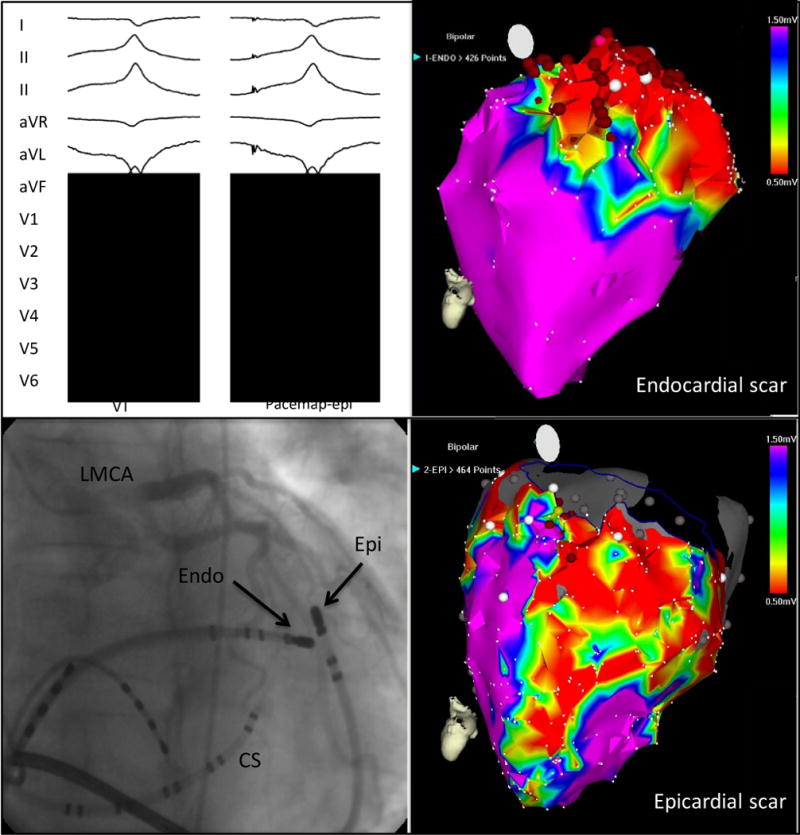
Epicardial Mapping and Ablation of VT. A 12-lead ECG of clinical ventricular tachycardia with a perfect pace map of the ventricular tachycardia from the epicardium. Top left, combined epicardial (Epi) and endocardial (Endo) mapping in the left anterior oblique projection (bottom left). A coronary sinus (CS) catheter is shown. Electroanatomic mapping demonstrates a greater extent of epicardial (bottom right) scar compared with endocardial scar (top right). Red circles represent areas of radiofrequency application. LMCA indicates left main coronary artery. (modified from Tung et al120, used with permission)
Table 1.
Epicardial VT Ablation Studies
| Author with ref# | Year | N | Substrate (n) | Epicardial Ablation (n) | Ablation Catheters | Ablation Approach | Acute Success | Follow-up (months) | Intermediate Success |
|---|---|---|---|---|---|---|---|---|---|
| Sosa25 | 1998 | 10 | Chagas CM | 6 | 4mm tip with temp to 60° | Pace mapping mid-diast or cont. EGMs | 100% | 4 to 9 | 100 |
| Sosa26 | 2000 | 14 | ICM (14) | 7 | NR | Thermal mapping mid dias. or cont. EGMs | 56% | 14±2 | 37% |
| Schweikert27 | 2003 | 30 | Normal heart (20) ICM (7) NICM (3) |
8 | Cooled Tip (Chilli or Thermocool) to 50°C mean 3+1 lesions | Activation mapping | 93% | 26±13 | NR |
| Sarabanda28 | 2005 | 56 | Chagas CM (56) | 56 | 8mm tip to 70°C | Ent or Pace mapping Presystolic EGMs targeted | 30% | NR | NR |
| Cesario29 | 2005 | 20 | ICM (12) NICM (8) |
6 2 |
8mm Navistar; 40–70W to 55° | Pace mapping Mid diast and fract. EGMs | NR | 12±4 | 75% |
| Daniels30 | 2006 | 12 | Normal | 12 | 4mmtip, 4mm irrigated, cryocatheter | Act., Pace mapping | 75% | NR | NR |
| Aryana31 (magnetic navigation) | 2007 | 24 | ICM (13) NICM HCM ARVC Sarcoidosis |
3 | 4mm tip (n=2); 4mm irrigated (n=22) | Act., Ent. Mapping Pace mapping Late and fract EGMs | 97% | 7±3 | 83% |
| Garcia32 | 2009 | 13 | ARVC (13) | 13 | 4mm tip RF 50W to 55°C.; Closed irrig.or open irrig. to 42° – both up to 50 W | Act. or Pace mapping; focal or linear lesions to LPs | 77% | 18±13 | NR |
| Schmidt33 | 2010 | 56 | Normal (16) ICM (11) NICM (16) ARVC (13) Myocarditis (3) |
12 10 12 10 1 |
Irrigated RF to 43° C and power to 50W median 1–12 lesions | Act and Ent mapping ‘Abnormal’ EGMs targeted | 69% 100% 76% 77% 66% |
12±5 | 47% |
| Sacher7 (multicenter) | 2010 | 134 | ICM (51) NICM (39) ARVC (14) Other CM (13) Normal (17) |
51 39 14 13 17 |
4mm, 8mm solid tip Open and closed cooled tip Cryocatheters power 20–50 W | Multiple | NR | 23±21 | 71% |
| Nakahara34 | 2010 | 33 | ICM (17) NICM (16) |
7 10 |
open irrigation (thermocool) 50W closed irrigation (Chilli) 8 mm tip (Navistar) | LP and fractionated EGMs | 82% (ICM) 44% (NICM) |
12±10 | 82% |
| Nadamanee35 | 2011 | 9 | Brugada (9) | 9 | 3.5 mm Thermocool ; 30–50W to 45 deg median 35 lesions | LPs and fractionated EGMs | 78% | 20±6 | NR |
| Bai36 (multicenter) | 2011 | 49 | AVRC (49) | 26 | 3.5 mm Thermocool | Act. or Ent mapping LPs and fractionated EGMs | 85% (endo. Abl. Only 52%) | 39±4 | NR |
| Della Bella37 (multicenter) | 2011 | 218 | ICM (85) NICM (67) ARVC (13) HCM (5) Normal (48) |
218 | Open irrig. Cath. (80% of cases) 20–40W, irrigation 25mL/min; Power titrated temp. <45°C or impedance drop <20 ohms. Non-irrigated RF catheters (12%); cryoabl(8%) | Pace or Ent. mapping | 72% | 17±18 | 68.6 |
| Dello Russo38 | 2012 | 20 | Myocarditis | 6 | Cooled tip with 40W to 43° | Act., Ent. Mapping Pace or Scar mapping | 100% | median 28 | 90% |
NR= Not Reported; Intermediate Success = Success Rate after the defined follow-up period Act.=Activation; Dias.=Diastolic; Fract.=Fractionated; Ent.=Entrainment; EGM=Electrograms; LP=Late Potentials;
The stimulus to the development of epicardial intervention came from the patient population with Chagas disease and VT. In their center, Sosa and colleagues found that VT was reproducible using programmed stimulation indicating a reentrant mechanism. Left ventricular mapping showed an inferobasal scar in 70% of patients as a likely substrate.2, 39 They observed fractionated ventricular EGMs in the coronary sinus catheter in some patients; however epicardial mapping was limited by venous anatomy. They also hypothesized that endocardial ablation might fail to create transmural lesions reaching epicardial circuits; hence the indication to investigate the epicardial approach.25
ECG Criteria Suggesting Epicardial VT (Figure 7)
Figure 7.
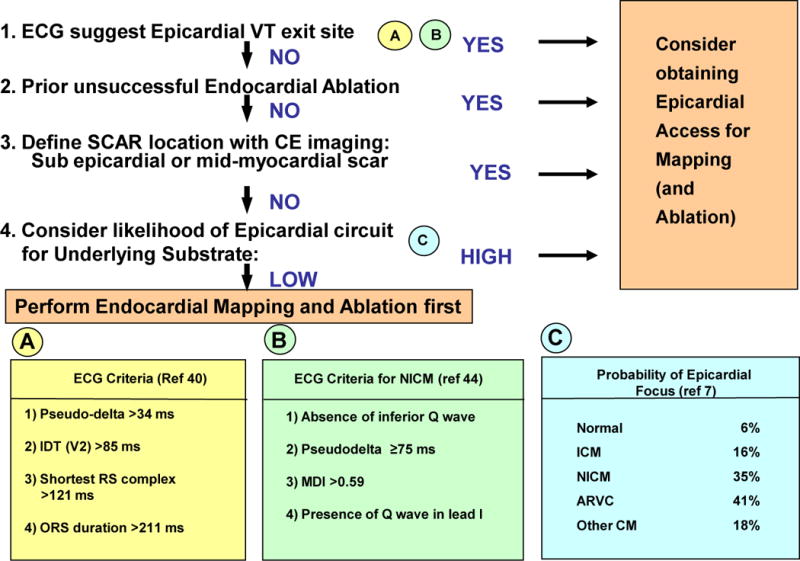
Flow Chart for Suggested Approach to Assessing Need for Epicardial Access and/or Ablation. CE = Contrast Enhanced; Pseudodelta = Interval from the onset of ventricular activation to the onset of the earliest rapid deflection in any precordial lead; IDT = Intrinsicoid Deflection Time is the interval from the onset of ventricular activation to the peak of the QRS in a precordial lead to the QRS duration; MDI = Maximal Deflection Index defined as the ratio of the interval from the onset of ventricular activation to the peak of the QRS in a precordial lead to the QRS duration.
Several groups have used their clinical studies to propose ECG criteria which would indicate an epicardial focus or circuit for VT. In 2004 Berruezo and colleagues reported criteria developed with endocardial and epicardial left ventricular (LV) pacing from a group of 9 patients, and further refined with three patient groups: a group successfully ablated epicardially after a failed endocardial approach, a second group successfully ablated endocardially and a third group who failed endocardial ablation.40 A pseudodelta wave interval (measured from the onset of ventricular activation to the onset of the earliest rapid deflection in any precordial lead) of >34 ms has a sensitivity of 83% and a specificity of 95%, an intrinsicoid deflection time (IDT; measured from the onset of ventricular activation to the peak of the R wave in lead V2) of > 85 ms has a sensitivity of 87% and a specificity of 90%, and an RS complex duration of > 121 ms has a sensitivity of 76% and a specificity of 85% in identifying an epicardial origin of the VT. The mean QRS duration was 217±24ms in the epicardial group versus 174±37ms in the endocardial group which was statistically significant, but no criterion based on QRS duration was defined; however all patients with QRS duration > 211 ms had an epicardial VT. Of note the majority of patients in this study had ischemic CM (>64% for each group). However the most recent analysis of these criteria for the ischemic substrate suggests that none of these or other criteria could reliably predict an epicardial VT focus.41 While amiodarone treatment resulted in a non-significant trend to QRS prolongation, no effect was seen with other antiarrhythmic drugs which were discontinued five half-lives prior to electrophysiologic testing. However in the presence of antiarrhythmic drugs, these criteria are likely even less sensitive and specific.
Bazan et al. developed ECG criteria for ventricular tachycardia arising from the epicardial right ventricle, and the epicardial left ventricle in the absence of myocardial infarction.42, 43 They found these criteria were region specific, with sensitivity/specificity varying from 14 to 99% and 20 to 94%. In 2010, Valles and co-workers assessed the above criteria for a population of non-ischemic cardiomyopathy patients using both endocardial and epicardial pacing and clinical ventricular tachycardias.44 Using a set of endocardial and epicardial pace maps from a series of 14 nonischemic cardiomyopathy (NICM) patients, a 4-step algorithm was developed, which was then validated in a cohort of 11 patients [with 14 epicardial (EPI) and 7 endocardial (ENDO) VTs]. The following criteria were developed: (i) absence of inferior Q waves, (ii) pseudo-delta >75ms, (iii) maximum deflection index (MDI; defined as the ratio of the interval from the onset of ventricular activation to the peak of the QRS in a precordial lead to the QRS duration) >0.59, (iv) presence of a Q wave in lead I. When these criteria were applied in a four step algorithm they yielded a sensitivity of 96% and a specificity of 93%. Of note most of the VTs in NICM appear to originate from areas of substrate scar in the superior and lateral areas near the mitral valve. For idiopathic epicardial left ventricular VT, arising remote from the sinus of Valsalva, an MDI >0.55 identified an epicardial site with a sensitivity of 100% and a sensitivity of 98.7%.30
Based on the findings of these studies, ECG criteria to identify epicardial VT appear to be substrate specific, with the Berruezo et al criteria applicable to ischemic substrates and the Valles et al criteria to nonischemic substrates,45 with markedly higher sensitivities and specificities for the idiopathic and nonischemic substrates compared to the ischemic substrate.
Chagas VT
In their initial series of patients with VT and Chagas disease, Sosa et al. found an epicardial circuit for 14 of 18 mappable VTs induced in ten patients.25 In 10 of these 14 VTs, the earliest site was epicardial and RF delivery at these sites resulted in VT termination in 4.8±2.9 seconds. None of the VTs were reinducible in the six patients who underwent epicardial ablation. Most of the VTs (82%) are related to a left sided inferobasal scar,28 and a common reentrant circuit involved the surviving tissue between the scar and the mitral isthmus.46
In a more recent report from this lab on 257 consecutive patients referred for VT ablation, the prevalence of epicardial VT was higher in Chagas disease patients(37%) compared with post-myocardial infarction patients(28%) and idiopathic dilated cardiomyopathy (24%).47
VT in Normal Hearts
In the study of Schweikert et al., 20 of the 30 patients with VT who underwent epicardial mapping had ‘normal’ hearts.27 Of these patients, 7 were ablated from the aortic root, 9 were ablated epicardially, 2 were ablated endocardially and 2 could not be ablated. For the 9 patients ablated epicardially, there were a total of 17 VTs with mean epicardial activation occurring 36±5 ms pre-QRS, requiring a mean of 3±1 epicardial lesions. Of particular note, for the 17 VT that were successfully ablated epicardially, successful sites were clustered at the atrioventricular or interventricular grooves, along the course of major epicardial vessels. Daniels and co-workers reported that an epicardial LV site of origin was found in 12 of 138 (9%) of patients referred for ablation of idiopathic VT; patients with Sinus of Valsalva VT were not included in the analysis.30 Eleven patients were successfully ablated, 5 via the coronary venous system, 4 with the percutaneous epicardial approach and two with a surgical approach. Of note, the sites of successful ablation were also clustered around the course of the middle cardiac vein and the anterior interventricular vein, similar to the findings of Schweikert and colleagues.
Ischemic VT
In the initial report of epicardial catheter ablation in patients with post myocardial infarction scar related VT, Sosa and colleagues reported their findings in a series of 14 patients.26 Epicardial access was obtained in all patients. A total of 30 VTs were induced, of which 18 were mappable and of these, 7 were interrupted with epicardial ablation (39% of mappable VTs). In epicardial mapping, the earliest sites were 87± 13ms before the QRS onset. The overall success rate of combined endocardial and epicardial ablation in this study was 37% with follow-up of 14±2 months.
Our group reported an initial experience in a combined population of ischemic (n=12) and non-ischemic (n=8) cardiomyopathy patients referred for VT ablation to a tertiary referral center.29 All patients underwent48–50 both endocardial and epicardial mapping; high density voltage maps of the substrate were performed and showed the patients with ischemic cardiomyopathy tend to have a 2–3 fold larger scar area endocardially than epicardially. Most VTs (89%) were hemodynamically unstable and a substrate modification based approach was used for ablation. For the total population, 75% of patients were free of either shocks or ATP at one year. (Video 9) Combined epicardial and endocardial mapping allows a comprehensive delineation of the substrate and allows ablation of later potentials in sinus rhythm.48–50 In a recent study we found ischemic cardiomyopathy patients tend to have a better outcome following catheter ablation for VT (82% nonrecurrence at 12 +/− 10 months of follow-up).49
Schmidt and colleagues from Hamburg recently reported on 59 patients with multiple different substrates who underwent combined endocardial and epicardial mapping, following prior unsuccessful endocardial ablation.33 Eleven patients had an ischemic substrate with a mean of 1.6 inducible VTs, and epicardial ablation was performed in seven patients. Using both activation mapping for tolerated VTs and a substrate guided approach for unmappable VT, complete success, defined as no inducible VT, was obtained in 55% of the ischemic patients. Partial success, defined as noninducibility of the clinical VT was obtained in the remainder of the patients. The site of successful ablation was endocardial in 4, epicardial in 4, and both endocardial and epicardial in 3 patients.
Nonischemic VT
Hsia and colleagues first reported that epicardial mapping in patients with NICM demonstrated abnormal electrograms (amplitude <1.8mV) in 20±12% of the total endocardial surface, usually located near the ventricular base in the perivalvular region, with a comparable area (<25%) of endocardial low voltage.51 We also found that patients with NICM had similar size areas of scar (voltage <0.5mV) on the epicardial and endocardial surfaces, in contrast to ischemic cardiomyopathy substrates where the endocardial scar area was nearly three times larger than the epicardial scar area29. Soejima et al. reported on a series of 28 patients with VT and a NICM substrate.52 Of these, 26 underwent endocardial and 8 epicardial electroanatomic mapping. Of the 19 VT circuit isthmuses identified by entrainment or pace mapping, 12 were associated with endocardial scar and 7 with an epicardial scar. Epicardial scar areas were also larger than endocardial scar areas in these patients. Ablation with an internally irrigated 4mm electrode successfully ablated six VTs with an average of ten RF applications, 35 Watts to 41° C.
In a recent study from the University of Pennsylvania, Cano et al. reported on 22 patients with VT and NICM who underwent combined endocardial and epicardial mapping for suspected epicardial VT.53 Epicardial VTs were targeted in 18 patients on the basis of activation/pacemapping or by targeting split or late potentials. During an average of 18 months of follow-up, there was no recurrence of epicardial VTs in 14 of 18 patients (78%). The mean epicardial area of low voltage (<1.0mv) was 55.3 ±33.5 cm sq. versus 22.9±32.4 cm sq. for the endocardial surface.
Myocarditis
Ventricular arrhythmias may be the initial presentation in myocarditis.54, 55 In the largest series to date, Dello Russo and coworkers recently reported a multicenter study of 20 patients with biopsy proven myocarditis and drug refractory VT, of which 5 patients presented with VT storm.38 The mean EF was 55%, and all patients underwent endocardial RF mapping and ablation with irrigated catheters. This was successful in 14 patients, while the remaining 6 (30%) were successfully ablated epicardially. Subsequently two patients died of acute heart failure unrelated to the VT. Contrast enhanced cardiac magnetic resonance (CE-CMR) may be particularly useful for identifying scar location in patients with acute myocarditis. In an MR study of patients with myocarditis presenting with heart failure or ventricular arrhythmias, De Cobelli et al. found that late enhancement with gadolinium was present in 84% of patients, equally distributed between mid-wall and sub-epicardial distributions.56
Sarcoidosis/Granulomatous diseases
In granulomatous cardiac disease, ventricular arrhythmias may also be the first clinical presentation.57 Koplan at al. described a series of eight consecutive patients with monomorphic VT due to cardiac sarcoidosis, with VT the initial manifestation in 5 of 8 cases.58 An average of 4±2 VTs were induced in each patient, with areas of low voltage in the RV in all patients, in the LV for 5 and in the epicardium in 2 patients who underwent epicardial mapping. Post-ablation, all but one patient remained inducible for some VT; however with at least six months of follow-up, 4 patients were controlled with immunosuppression and antiarrhythmic drugs. Four patients eventually required cardiac transplant because of recurrent VT. Jefic and colleagues followed a multicenter registry of 42 patients with cardiac sarcoidosis who developed VT. In 9 patients, VT was not controlled with medical therapy (steroids and antiarrhythmic drugs), and RF ablation was performed for this group.59 A total of 44 VTs were induced in these nine patients, and the most frequent VT circuit was reentry in the peri-tricuspid area. One patient required epicardial ablation of a lateral LV site and had no recurrence. In total, 5 of the 9 patients who underwent ablation had no recurrence at 20 months of follow-up.
Arrhythmogenic Right Ventricular Cardiomyopathy (ARVC)
In the first complete description of ARVC in 1982, a ‘triangle of dysplasia’ involving the inferior RV, the RV apex and the right ventricular outflow tract (RVOT) was defined,60 and within a year Guiraudon reported a surgical treatment of ventricular tachycardias in these patients by total disconnection of the RV free wall.61 Marchlinski and colleagues confirmed that patients with ARVC) have a predilection for scar in the basal perivalvular, apical and outflow tract areas using epicardial mapping.62 In a study of 13 patients who underwent endocardial and epicardial mapping after failed endocardial ablation, this group reported that areas of low voltage (< 1 mV for epicardium and 1.5 mV for endocardium), was more extensive on the epicardium and demonstrated multicomponent and late potentials.32 Twenty-seven VTs were targeted on the epicardium, which were opposite ineffective endocardial ablation sites in 11 patients. The approach combined activation, entrainment or pace mapping with focal or linear lesions targeting late potentials. During an average 18 months of follow-up, 10 of 13 patients (77%) had no VT. In a recent multicenter study, Bai et al. compared two groups of AVRC with VT who underwent endocardial only ablation (n=23) or endo-epicardial ablation (n=26).36 The ablation strategy was to target all sites with fractionated or late potentials seen on electroanatomical mapping. After at least three years of follow-up, freedom from ventricular arrhythmias or ICD therapy was 69.2%(18/26) in the endocardial ablation only group and 84.6% (22/26) in the combined endo-epicardial ablation group. The authors concluded that a combined endo-epicardial ablation strategy was superior for VT ablation in arrhythmogenic right ventricular dysplasia/cardiomyopathy patients. More recently, Berruezo et al. reported similar results with a combined epicardial endocardial approach in 11 patients with ARVC, with a recurrence rate of 9% at 11 months of follow-up.63
Brugada Syndrome
Epicardial mapping studies by Nagase and colleagues showed that the negative T wave associated with type I Brugada ECG pattern was associated with a prolongation of the AP in the epicardium compared with the endocardium in the region of the RVOT.64 Recently Nademanee and coworkers reported a new application of epicardial ablation to patients with type I Brugada syndrome and recurrent episodes of ventricular fibrillation (VF).35 The study reported on 9 patients with a median four episodes of VT per month requiring ICD shocks. Combined endocardial and epicardial mapping of the right ventricle revealed areas of abnormal low voltage(0.94±0.79 mV) with prolonged duration and fractionated EGMs localized to the anterior aspect of the RVOT epicardium. Ablation of these sites rendered VT/VF noninducible in 7/9 patients (78%), with no clinical recurrences in these patients during a mean follow-up of 20 months. Additionally, following ablation, the ECG pattern normalized in 8/9 patients (89%). This study supports the hypothesis that abnormal delayed depolarization is the mechanism of VT/VF in Brugada patients with an arrhythmogenic substrate localized to the RVOT epicardial region.
Current State of the Art: European Multicenter Study 2011
Della Bella and colleagues reported outcomes from six tertiary VT referral centers, (with an experience of at least eight epicardial cases) in Europe in 2011.37 The analysis included 218 patients who underwent epicardial mapping (87% had concurrent endocardial mapping). Catheter ablation was attempted for 289 induced VTs with a strategy based on pace or entrainment mapping and targeting pre-systolic or mid-diastolic potentials. RF energy was delivered on the epicardial surface only in 103VTs (35.6%), on the endocardial surface only in 41VTs (14.2%), and both endocardially and epicardially for 145VTs (50.2%). After a mean follow-up of 17 months, the overall recurrence rate was 31.4%; this was not significantly different for the various cardiac substrates: nonischemic cardiomyopathy [NICM] (39.3%), ischemic cardiomyopathy [ICM] (33.8%), arrhythmogenic right ventricular cardiomyopathy [ARVC] (30.8%), hypertrophic cardiomyopathy [HCM] (25%) and idiopathic ventricular tachycardia (17.1%). Twenty patients (10.4%) died during the follow-up period (12 of heart failure, 2 of cardiac arrest, and 6 of non-cardiac causes), while 3 patients underwent heart transplantation.
Epicardial Ablation using Magnetic Navigation
Aryana et al. reported on the use of remote magnetic navigation to guide endocardial and epicardial mapping and ablation for scar related VT.31 In 15 of 24 patients the substrate was ICM, with substrates of NICM, HCM, ARVC and sarcoidosis in the remainder. Electroanatomical maps using the Stereotaxis system were constructed for the left ventricular, right ventricular and epicardial surface in 24, 10 and 12 patients respectively. A total of 77 VTs were targeted, with a manual irrigated catheter used for ablation in 22 procedures; 75 of 77 (97%) of VTs were eliminated. For 21 hemodynamically stable VTs targeted for ablation during VT in 15 patients, 17(81%) were successfully terminated; for these 15 patients, the successful sites were LV (for ICM and NICM), RV for the sarcoidosis patient and epicardial RV for the three ARVC patients. Four patients underwent a second procedure, 3 with magnetic navigation; there were no VT recurrences during a mean of 7±3 months of follow-up.
Surgical Approach to Epicardial VT Ablation
First developed in the 1970s with the subendocardial excision method for recurrent ventricular tachycardia65, but largely abandoned by the 1990s because of high procedural mortality, surgical guided ablation has recently made a comeback with the development of the percutaneous epicardial approach, electroanatomic mapping techniques and advances in catheter technology. In our series of 14 patients who underwent a surgical approach for epicardial access, 10 had an ischemic cardiomyopathy substrate.23 Of these ten, 8 underwent epicardial ablation using a closed irrigation catheter with power settings from 20–50W, titrating for a target temperature of 42° C and monitoring for an impedance drop. We have also reported on a patient with ischemic cardiomyopathy and an LVAD device, who underwent anterior thoracotomy for recurrent ventricular tachycardia after unsuccessful endocardial ablation.66 The epicardial access allowed ablation using a surgical ablation tool (Isolator Coolrail, Atricure Inc., West Chester, OH) composed of two 30mm electrodes, with bipolar RF delivered between the two electrodes and internal irrigation. This allowed delivery of RF to a broad region, with lesion depth to 4–5mm, and successful ablation of the clinical VT.
Anter at al. recently reported a series of 8 patients with nonischemic cardiomyopathy and refractory ventricular tachycardia despite prior endocardial (n=8) and epicardial (n=6) ablation.67 After an unsuccessful percutaneous approach, median sternotomy was performed and surgical cryoablation was delivered directly at sites previously identified by electroanatomic mapping and pacing techniques. Two patients died in the post procedure hospitalization period due to heart failure and sepsis, while in the remaining six patients the median time from surgery to discharge was 7 days. Over a mean follow-up of 23±6 months, 4 patients remained free of VT and the number of implantable cardioverter defibrillator (ICD) shocks per patient declined from 6.6 in the three months before surgery to 0.6 in the three months after surgery. Surgical ablation tools have also been used in this setting in the electrophysiology (EP) lab for creating ablative lesions.66
The surgical option offers several advantages when dealing with epicardial or mid myocardial circuits that cannot be reached with endocardial or epicardial catheter ablation.68 Surgical cryoablation delivers temperatures to −160° C achieving deeper lesions than catheter cryoablation, while direct visualization of the myocardium allows avoidance or mobilization of the coronary arteries and dissection of epicardial fat. As the authors point out, these patients comprise approximately 5% of the patients referred for VT ablation at the severe end of the spectrum, and perioperative mortality remains a concern. Nevertheless, this is now a viable option for these select patients, with an experienced cardiac EP and cardiac surgical team.
When to Perform Epicardial Access and Ablation (Figure 7)
Multiple factors need to be considered when deciding whether an epicardial approach is needed.69 A suggested approach is outlined in Figure 7. This includes ECG analysis of the clinical VT for features suggesting epicardial source, history of a prior failed endocardial ablation, contrast enhanced computed tomography (CT) or magnetic resonance (MR) imaging suggesting a sub-epicardial or mid-myocardial scar and an estimation of the likelihood of epicardial VT given the cardiac substrate. It is important to make this decision before starting the case, as planning and logistics are very important in an epicardial access case. In our lab, we obtain epicardial access immediately after placing the standard intracardiac catheters; His bundle (His), right ventricle (RV), coronary sinus (CS) and before accessing the left ventricle via the transseptal route. After epicardial access is obtained, and there is no evidence of epicardial bleeding or complications, the patient is fully heparinized and endocardial left ventricular (LV) access is obtained by the transseptal approach (our preferred approach to avoid aortic transit of catheters). We then proceed with epicardial and endocardial voltage mapping.
Epicardial Supraventricular Tachycardias (Table 2)
Table 2.
Epicardial SVT Ablation Studies
| Author with ref#. | Year | N | Substrate/Epicardial Access (n) | Epicardial Ablation Performed (n) | Catheter | Ablation Target | Follow-up (months) | Intermediate Success |
|---|---|---|---|---|---|---|---|---|
| Katritsis70 | 2001 | 40 | Paroxysmal AF (14) | 2 | 25W to 50°C | Activation mapping | NR | NR |
| Schweikert27 | 2003 | 18 | Pathway (10) IST (4) AF/AFL (4) |
3 1 0 |
Irrigated tip to 50°C | Activation mapping | 26±13 | 66% |
| Valderrabano71 | 2004 | 6 | Pathway (6) | 2 | 50W at 60–70°C | Activation mapping | 4 | 84% |
| Scanavacca72 | 2006 | 10 | AF (7) | 7 | 30–50W to 60°C | Evoked Vagal reflex mapping | 8 | 30% |
| Pak73 | 2007 | 5 | AF (5) | 5 | Open irrigated; 35W to 50° | PV isolation | 8±6 | 100% |
| Phillips74 | 2008 | 2 | LAA AT (2) | 2 | 4mm; 50W to 50° | Activation mapping | NR | NR |
NR= Not Reported; Intermediate Success = Success Rate after the defined follow-up period
AF=Atrial Fibrillation; AFL=Atrial Flutter; AT=Atrial Tachycardia; IST=Inappropriate Sinus Tachycardia; LAA= Left Atrial Appendage; PV=Pulmonary Vein
The epicardial approach is less commonly used for supraventricular tachycardia (SVT) ablation than for VT ablation, and almost always used after a patient has already undergone an unsuccessful endocardial ablation. SVT’s successfully treated with this approach include accessory pathways, atrial tachycardia and atrial fibrillation.
Accessory Pathways (Fig 871 & video 8)
Figure 8.
Epicardial Catheter Ablation of a Right Sided Accessory Pathway. Combined epicardial-endocardial AP mapping of a right-sided AP. Panel A, Electrograms during orthodromic AV tachycardia, showing earliest atrial activation in the epicardial catheter (vertical line). RF delivery abolished retrograde AP conduction during ventricular pacing (right panel). Panel B: Left anterior oblique fluoroscopic view of the catheters. HRA, high right atrium; EPI, epicardial catheter through an long sheath; ENDO, endocardial catheter; RCA, right coronary artery; HIS, His bundle catheter; RV, right ventricular catheter; CS; coronary sinus catheter; Halo, tricuspid annulus catheter. (modified from Valderrabano et al71)
The first reported ‘ablation’ of an accessory pathway was using the open surgical approach in 1969.5 With the development of endocardial RF catheter ablation, most bypass tracts became accessible to catheter ablation. While the coronary sinus,75, 76 or a coronary sinus diverticulum,77, 78 may provide a route to locate and ablate epicardial pathways, there are some pathways that cannot be reached via the coronary sinus or its branches. Reported cases indicate a wide variety of pathway locations including left posterolateral and posteroseptal, midseptal, right sided and right atrial appendage to right ventricle. In some cases, a combined epicardial and endocardial approach has been successfully used for resistant pathways when endocardial or epicardial approaches alone were unsuccessful.71
Epicardial pathway location has been cited as a reason for failed endocardial ablation in 8% of cases.79 Schweikert and coworkers reported on a series of 10 patients with accessory pathways and previously failed endocardial ablations.27 The group included both manifest and concealed pathways, with four left sided, one midseptal and five right sided pathways. In five cases, the earliest activation was recorded epicardially; three were right atrial appendage to RV and were successfully ablated, while a right posteroseptal and left posteroseptal pathway were successfully ablated endocardially despite an earlier epicardial site. In the case of the left postero-septal pathway, there was high impedance with epicardial ablation, possibly due to epicardial fat. During a two year follow-up, one patient had a recurrence and was successfully re-ablated. There were no procedure related complications, and there was no difficulty in re-accessing the pericardial space.
Our group has reported on a series of six patients with previously unsuccessful endocardial ablation of accessory pathways referred for epicardial ablation.71 Six patients with 7 APs were studied; six of the pathways were right sided, and of these, five were located in the right free wall. All patients also underwent simultaneous endocardial mapping. The successful ablation site was endocardial in one patient, epicardial in four and simultaneous epicardial and endocardial in one patient.
In a series of 89 cases of WPW referred to tertiary centers after initial unsuccessful ablation attempts80, the most common reasons for failure were catheter manipulation problems and inadequate mapping. An irrigated tip catheter was required for epicardial pathways (n=7), of which 6 were ablated from the coronary sinus and one via percutaneous epicardial access.
Atrial Tachycardias
Successful epicardial ablation has also been reported for atrial tachycardias, specifically inappropriate sinus tachycardia (IST) and atrial tachycardias (AT), particularly those arising from the left atrial appendage. Schweikert reported four cases in his series of SVT cases; one of the two with an earliest epicardial site was ablated endocardially.27 While this arrhythmia did not recur, overall there was a 50% recurrence rate for IST. A combined epicardial-endocardial approach was used for another reported case of IST.81 Phillips et al. reported two cases of atrial tachycardia arising from the left atrial appendage that were successfully ablated epicardially following a failed endocardial approach.74 The complex anatomy of the atrial appendage, which is also more prone to perforation from mechanical pressure, could make the epicardial route the preferred approach for atrial appendage tachycardias.
Atrial Fibrillation (AF) (Fig 982)
Figure 9.
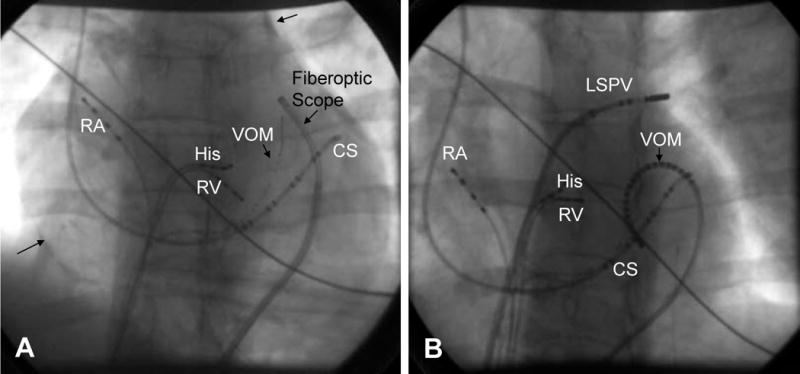
Fiberoptic Pericardial Imaging, Epicardial Mapping and Ablation of the Vein of Marshall. (modified from ref 82). Panel A: Fiberoptic scope in the pericardium, RA=right atrial catheter, His= His bundle catheter, CS= coronary sinus catheter, RV=right ventricular catheter, VOM= vein of Marshall Catheter (1.5 French, multipolar catheter in the vein introduced via the CS). Arrows show contrast in the pericardial space. Panel B: Epicardial mapping of the Vein of Marshall, in this case a multi-electrode VOM catheter has been placed in the pericardial space. RA=right atrial catheter, His= His bundle catheter, CS= coronary sinus catheter, RV=right ventricular catheter, VOM= multi-electrode vein of Marshall Catheter
There is very limited data on ablation of atrial fibrillation using an epicardial approach. The reflections of the visceral pericardium prevent complete encircling lesions of the pulmonary veins being achieved by this approach.82 The vein of Marshall, in some cases, may be a trigger site for induction of atrial fibrillation, and can be approached both endocardially via the coronary sinus or epicardially. Hwang and coworkers reported on a series of 28 patients undergoing AF ablation; in 6 patients direct vein of Marshall recording documented the origin of the AF in the muscle bundle within the ligament of Marshall.83 RF ablation at the insertion site successfully terminated AF in 4 of these 6 patients. In a study of forty patients undergoing catheter ablation for paroxysmal atrial fibrillation, catheterization of the vein of Marshall via the coronary sinus was feasible in 14 of 19 patients with foci in the left superior or inferior pulmonary veins.70 In 2 patients the sole focus was epicardial and was ablated epicardially via the coronary sinus.
Pak and colleagues reported a series of 5 patients who relapsed after endocardial AF ablation; additional epicardial ablations at pulmonary vein adjacent sites resulted in successful pulmonary (PV) isolation.73 In a recent report on epicardial mapping in patients undergoing a surgical Maze procedure within 8+11 months of an endocardial ablation, 15 of 24 (62.5%) of the pulmonary veins examined showed conduction on the epicardial regions.84 Choi et al. reported a hybrid endocardial-epicardial procedure in a patient with an anterior atrial wall mass guided by CT merged with 3D electroanatomical mapping.85 In a recent case of persistent atrial fibrillation, a combined endocardial-epicardial approach was used to create a box-set of lesions on the posterior atrial wall isolating all four PVs and the posterior wall together.86
Scanavacca and coworkers examined a strategy of targeting atrial sites in which high frequency stimulation (20Hz) induced vagal reflexes (AV block >2 seconds) in ten patients with paroxysmal AF and no heart disease.72 Endocardial and/or epicardial RF ablation was performed at these sites in 7 patients, while pulmonary vein isolation (PVI) was performed in 3 patients in whom evoked vagal reflexes were not obtained. AF recurred at 8 months in 5 of the 7 patients (71%) who underwent the vagal selective guided ablation and none of the PVI patients. Given the small number of patients, further studies are needed to define if this approach has a role in AF ablation procedures.
While the epicardial approach is appealing for ablation of atrial fibrillation, potentially avoiding the complications of embolic events and tamponade, these advantages must be balanced against the increased risk of damage to the coronary arteries, phrenic nerves and esophagus. Approaches adopted to protect of the phrenic nerves and esophagus include the use of balloons, saline and air in the pericardial space. As with ablation of epicardial ventricular circuits, the presence and extent of epicardial fat may prevent effective ablation of epicardial pathways.
Collateral Injury and Complications (Fig 10)
Figure 10.
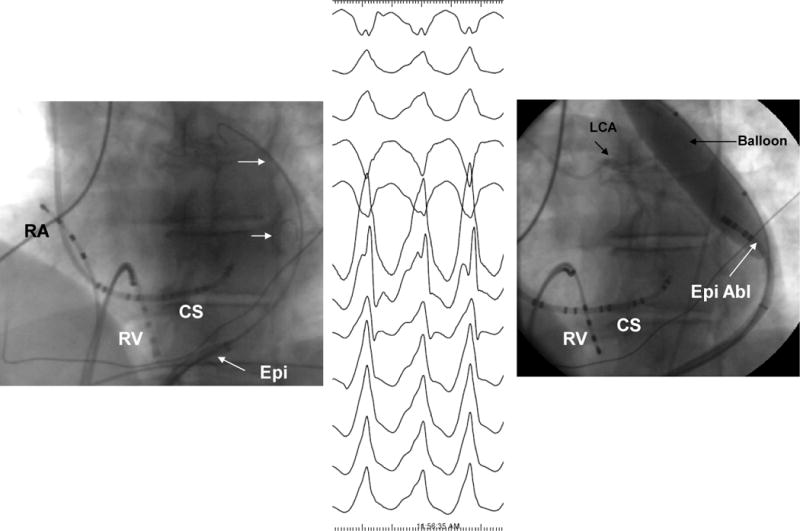
Phrenic Nerve Protection by Intrapericardial Balloon Placement: Double pericardial wire technique and pericardial balloon placement. RV= right ventricular catheter, CS=coronary sinus catheter, Epi+ epciardial sheath, epi abl= epicardial ablation catheter, LCA= left coronary arterial injection
As would be expected, entering, mapping and ablation in the pericardial space is associated with multiple potential complications. When the pericardial space is accessed, the liver, colon, and diaphragm with its vascular supply may be injured. If the needle may be advanced too far or too fast, it may enter the right ventricle, the pleural space or the lung. Use of the curved tip Tuohy needle will help decrease the risk of entering the RV and direct the guide wire when the epicardial space is entered (Fig 2 and appendix). Knowledge of the anatomy of the subxiphoid space and the pericardial space, and an awareness of potential complications is essential.
In their initial description of the epicardial access technique in a series of three patients, Sosa and colleagues reported no complications, and specifically noted that echocardiograms one day post the procedure showed no pericardial effusion.1 In a recent summary of their group experience, the most common complications were related to puncture accidents.87 A ‘dry’ right ventricular puncture occurred in 4.5% of 215 consecutive cases, and a hemopericardium requiring drainage was seen in 7% of cases (200±100ml. blood). Avoiding coronary artery damage is a major concern while ablating in the epicardial space. A coronary angiogram will show the location of the coronary arteries with respect to proposed ablation site. Radiofrequency (RF) energy is not delivered unless the distance between the catheter tip and a visible coronary artery is at least 1 cm, which is based primarily on operator experience,2 and on limited studies.88 In only 1 of their 215 consecutive patients did RF delivery result in occlusion of a coronary marginal branch, resulting in a non-Q wave myocardial infarction (MI) and a peak creatine kinase (CK) of 35U/L; however more data is needed to further clarify this important concern. By using a high output pacing (15mA, 5msec pulse) to test at ablation sites possibly close to the phrenic nerve, they had no cases of phrenic nerve injury. Epicardial fat with thickness of > 5mm may insulate the myocardium and attenuate RF lesion formation, even with cooled tip catheters. Pericarditis without pericardial effusion was seen in 30% of the patients post procedure, which responded to anti-inflammatory drugs. In a group of 29 patients who had a repeat epicardial access, no adhesions were found.
Sacher and colleagues reported a multicenter safety study from three tertiary centers performing epicardial VT ablation in 2010.7 VT ablation required epicardial access in 121 (13%) of 913 procedures. There were 8 (5%) acute major complications related to epicardial access – 7 epicardial bleeding and 1 coronary stenosis. Three delayed complications occurred after 48 hours: one major pericardial inflammation, one tamponade (ten days) and one acute inferior myocardial infarction (two weeks). No constrictive pericarditis or phrenic nerve injuries were reported. There were six complications related to concomitant endocardial ablation: pulmonary embolism (2), pericardial effusion(1), infranodal AV block (1) and groin hematomas requiring blood transfusion (1).
In the European multicenter study reported by Della Bella in 2011,37 major complications were observed in 9 (4.1%) of the patients, (cardiac tamponade in 8 and abdominal hemorrhage in 1). Complications classified as minor, were observed in 17(7.8%) of the patients, including vascular, heart failure, intermittent atrioventricular block and pneumonia. Ten percent of patients experienced severe post procedural pericardial pain.
A recent report highlights a series of unusual complications associated with percutaneous epicardial access in a multicenter series of 334 patients in a five year period89. These included sub capsular hepatic hematoma, coronary spasm and right ventricular pseudo aneurysm which were all managed conservatively. Surgical intervention was required for cases of liver puncture with intra-abdominal bleeding, pericardial bleeding due to middle cardiac vein laceration, pericardial bleeding due to coronary sinus laceration and right ventricular abdominal fistula. Other uncommon complications such as a pleuropericardial fistula may occur, and should be considered if a new left pleural effusion occurs after the procedure.90
Recently it has been shown that repeat pericardial access can be performed safely at a median of 110 days after the initial procedure in a series of 30 patients.91 Adhesions were encountered in 7 patients, and were easily divided with blunt dissection by a deflectable catheter in 5 of the 7 patients. A 90% acute success rate for the targeted VT was achieved in this study. However, the same center has also just reported a case of constrictive pericarditis after a fourth epicardial ablation procedure, resulting in severe heart failure, requiring a pericardiotomy.92
Methods to Reduce Complications
Several approaches have been developed to minimize or prevent these epicardial ablation related complications. As symptoms of pericarditis can occur in up to 30% of patients,2 nonsteroidal anti-inflammatory medications are usually given post procedure. Additionally, injection of glucocorticoid into the pericardial space also reduces inflammation in an animal model,93 and is now routinely used in our center, where we give methylprednisolone 250mg intra-pericardially at the end of the procedure.
Multiple techniques have also been developed for phrenic nerve protection. Our group has adapted a Meditech balloon (Boston Scientific, Natick, MA) to displace the phrenic nerve and protect it during RF ablation on the lateral epicardial wall.94 (Fig 7, Video 11 and 12) Fan et al. reported that in a series of ten patients with NICM undergoing epicardial VT ablation, 7 had evidence of phrenic nerve capture near targeted ablation sites.95 In one patient a peripheral angioplasty balloon, 18×60mm) was used to displace the phrenic nerve, while adjacent sites without phrenic nerve capture were ablated in the other patients. Di Biase et al. compared multiple methods for separating the phrenic nerve from the epicardial surface during epicardial RF ablation.96 These were: (1) placing a large size (25×40 mm.) peripheral angioplasty balloon in the pericardial space, (2) introducing saline in 20 ml. increments, (3) introducing air in 20 ml increments and (4) introducing a combination of air and saline. At each step epicardial pacing was performed to look for phrenic nerve stimulation. The saline or air infusions were increased until there was loss of phrenic capture or if the systolic blood pressure dropped below 60 mmHg. Saline failed in all cases, air alone failed in 6 of 8 cases, the balloon could not be accurately placed or failed in 6 of 8 cases, while the air+saline combination was successful in 7of 8 cases. The authors concluded that inflation with air and saline together provided the best strategy for preventing phrenic nerve injury. The intra-pericardial balloon approach can also be used for esophageal protection during ablation of the posterior wall in atrial fibrillation ablation as described by Buch et al.97 Significant reductions in esophageal temperature were shown in an animal model utilizing this method of esophageal protection.98 A similar approach has also been used to protect the right phrenic nerve during ablation of atrial tachycardias arising along the crista terminalis.99
Protection of the coronary arteries is a key concern when ablating in the epicardial space.100 There is little data available in addition to the empiric experience of Sosa and colleagues discussed above. D’Avila et al. studied the effects of RF lesions delivered in the vicinity of coronary arteries,101 and found that the risk of vascular damage varied inversely with vessel size. Larger vessels with internal diameter > 0.5 mm demonstrated matrix proliferation in the media, but no intimal proliferation, while severe hyperplasia or endovascular thrombosis was seen in vessels <0.5mm. This implies that larger vessels are protected by increased blood flow and possibly epicardial fat in addition. In a study of excised sheep hearts, Thyer at al. studied the effects of instillation of intracoronary chilled saline (5° C) during RF ablation over the coronary artery.102 The peak intracoronary temperature was 23.6 °C. in the chilled versus 54.6 °C. in the non-chilled vessels, while the median distance between the lesion and the artery was 0.42 mm versus 0.0 mm respectively. Viles-Gonzalez and co-workers delivered RF lesions within 1 mm of the coronary arteries in seven pigs, and examined the acute (20 days) and chronic (70 days) effects.103 In both the acute and chronic specimens, intimal and medial thickening was seen, with replacement of smooth muscle cells by extracellular matrix. No significant stenosis was observed up to 7 days after ablation. It remains to be seen whether these findings can be applied clinically in the EP lab; for now the best approach appears to be to avoid RF delivery within 1 cm of an epicardial coronary artery.
Finally, new approaches to the pericardial space and new devices may also lessen procedure related complications. Scanavacca and colleagues recently described a percutaneous transatrial approach where the epicardial space was accessed via the atrial appendage.20 The epicardial space was successfully entered in 17 animals and epicardial catheter manipulation performed. Hemodynamic compromise occurred in two animals where the puncture was outside the atrial appendage (RV in one and tricuspid annulus in another). In a study of the pressure–frequency patterns in the thorax and pericardium, Mahapatra and coworkers found a distinctive two peak pattern in the pericardial space, which may aid development of new access tools.104 These workers have also designed a novel percutaneous access needle which draws the pericardium away from the surface of the heart prior to engagement, thus minimizing the risk of ventricular perforation.105
Energy Sources/Special Considerations for Epicardial Ablation
To date, reported clinical studies have primarily used RF as the energy source for epicardial ablation. Lesion formation with standard RF catheters in the pericardial space will be limited by lack of convective cooling without circulating blood, resulting in high electrode temperatures at low power settings. D’Avila and colleagues compared the efficacy of standard versus cooled-tip RF catheters for epicardial ablation in goat and pig animal models with healed myocardial infarctions.106 Standard RF lesions using a 4mm tip catheter were 3.7±1.3mm (25±16.8 W), while cooled tip RF lesions were 6.7±1.7mm (44.8±6.8W). In areas covered by epicardial fat > 3mm, standard RF did not generate any appreciable lesions, while the cooled-tip RF lesions were 4.1±2 mm in depth (45±4.4W). The authors concluded that cooled tip RF could create epicardial lesions more effectively than standard RF ablation. During RF ablation with open irrigation catheters, the irrigation flow may be up to 30ml/min., which will require intermittent drainage of the pericardial space. Theoretically, the absence of circulating blood in the pericardial space should be advantageous to the use of cryoablation. Di Biase et al. reported two cases, one an atrial tachycardia and the second a ventricular tachycardia, where cryoablation was ultimately successful when RF ablation had failed.107 However, as shown by Lustgarten and colleagues, cryoablation may cause epicardial vessel damage by neointimal proliferation, with the probability of vessel damage inversely proportional to the vessel diameter.108 Suggested explanations included presence of epicardial fat or better adherence of the cryocatheter. However the mechanisms are still not clear and size of the catheter tip may play a an important role109. Other potential energy sources include microwave, laser and ultrasound.110 Epicardial mapping also needs to take into consideration epicardial fat which can mimic low voltage areas due to the insulating effects of fat. Electrogram characteristics can help delineate differences between true scar versus fat causing a reduction in recorded voltage.111
Emerging Pericardial Procedures: Percutaneous Atrial Appendage Closure
In non-valvular atrial fibrillation, up to 90% of thrombi originate from the left atrial appendage.112 Left atrial appendage occlusion devices are available, but require trans-septal catheterization with associated complications including air embolism, bleeding, tamponade and device embolization requiring surgical removal.113, 114 These problems have been the impetus for the development of new methods of left atrial occlusion using the percutaneous epicardial approach. Friedman and colleagues reported preliminary results using a novel left atrial appendage closure device applied via a single percutaneous sheath in 4 dogs.115 The device consists of a grasping tool which can record left atrial appendage (LAA) activity and which holds the appendage while a pre-loaded suture loop is advanced over the appendage to the base and fixed in place occluding the mouth of the appendage. The device was able to achieve appendage closure in all the animals as confirmed by amputation of the appendage in 2 animals and necropsy in all animals. This method has been further modified using a transseptally placed endoluminal balloon in the LAA with a magnet tipped guide wire and a magnet tipped wire of opposite polarity in the epicardial space (LARIAT, Sentre Heart, Palo Alto, CA) [‘Lariat’ refers to a type of knot or lasso].116 When the magnets are in proximity they align, allowing traction on the appendage as the snare loop is advanced to the base of the LAA. The endovascular guide wire and balloon are then detached, and the suture is tightened around the base of the LAA. In five animals where the endocardial balloon was used, the level of the LAA closure was at the base, while this occurred in only 1 of 4 when it was not used. In all animals LAA closure was complete without a leak. Lee and coworkers studied 26 dogs using this device.117 Sixteen dogs were euthanized and found to have the base of LAA closed in all cases; in the remaining animals, examination of the LAA at 1 week, one month and 3 months demonstrated a completely endothelialized origin of the LAA. In the first study in humans, Bartus at al. studied thirteen patients, either undergoing mitral valve surgery (n=2) or atrial fibrillation ablation(n=11).118 Both of the mitral valve repair (MVR) patients had complete closure of the LAA determined by visual inspection, while this was achieved percutaneously in ten of the 11 AF patients, with one patient requiring a thorascopic procedure to remove the snare due to pectus excavatum. The procedure was terminated in one patient due to poor echocardiographic visualization of the marker balloon. Sixty day follow-up was reported for 6 patients – four had complete LAA closure, while two had a 2mm opening by color flop Doppler. The authors concluded that catheter based suture ligation of the LAA is feasible in humans, with further investigation needed to determine long term safety and efficacy.
Conclusions
In the last fifteen years, interventional cardiac electrophysiology has ventured into the pericardial space with notable added benefits for treatment of arrhythmias, particularly for ventricular tachycardia. Additionally, the ability to access the epicardial surface of the heart has enabled mechanistic studies regarding arrhythmia mapping and substrates.34, 119 Advances in epicardial therapies for atrial arrhythmias have been slower, but presents an opportunity to emulate the achievements in treating ventricular arrhythmias.
New methods to make pericardial access easier and a lower risk procedure are being developed. Protection of the coronary arteries and surrounding structures such as the esophagus and phrenic nerves will remain an important feature when working in this space. Future developments in cardiovascular science could create the need for ‘site-specific’ epicardial delivery of biological therapies.
Supplementary Material
Acknowledgments
The authors would like to dedicate this review to Drs. Eduardo Sosa, Mauricio Scanavacca, Andre D’Avila, and their EP and cardiology colleagues for their pioneering work on epicardial ablations at the Incor Institute, Sao Paulo, Brazil. Their generosity in hosting and training several teams around the world in this approach is also appreciated. We would also like to thank our colleagues, staff and trainees in the past decade who have all participated in these procedures as they evolved at our center.
Funding Sources: Supported by the NIH/NHLBI (National Heart Lung and Blood Institute) (R01HL084261 to KS)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: The University of California, Los Angeles has intellectual property developed by the authors that relate to epicardial interventions.
References
- 1.Sosa E, Scanavacca M, d’Avila A, Pilleggi F. A new technique to perform epicardial mapping in the electrophysiology laboratory. Journal of cardiovascular electrophysiology. 1996;7:531–536. doi: 10.1111/j.1540-8167.1996.tb00559.x. [DOI] [PubMed] [Google Scholar]
- 2.Sosa E, Scanavacca M. Epicardial mapping and ablation techniques to control ventricular tachycardia. Journal of cardiovascular electrophysiology. 2005;16:449–452. doi: 10.1046/j.1540-8167.2005.40710.x. [DOI] [PubMed] [Google Scholar]
- 3.Sosa E, Scanavacca M. Images in cardiovascular medicine. Percutaneous pericardial access for mapping and ablation of epicardial ventricular tachycardias. Circulation. 2007;115:e542–544. doi: 10.1161/CIRCULATIONAHA.107.701623. [DOI] [PubMed] [Google Scholar]
- 4.Guiraudon GM, Klein GJ, Sharma AD, Jones DL, McLellan DG. Surgery for Wolff-Parkinson-White syndrome: further experience with an epicardial approach. Circulation. 1986;74:525–529. doi: 10.1161/01.cir.74.3.525. [DOI] [PubMed] [Google Scholar]
- 5.Sealy WC, Hattler BG, Jr, Blumenschein SD, Cobb FR. Surgical treatment of Wolff-Parkinson-White syndrome. The Annals of thoracic surgery. 1969;8:1–11. doi: 10.1016/s0003-4975(10)66402-8. [DOI] [PubMed] [Google Scholar]
- 6.Aliot EM, Stevenson WG, Almendral-Garrote JM, Bogun F, Calkins CH, Delacretaz E, Bella PD, Hindricks G, Jais P, Josephson ME, Kautzner J, Kay GN, Kuck KH, Lerman BB, Marchlinski F, Reddy V, Schalij MJ, Schilling R, Soejima K, Wilber D. EHRA/HRS Expert Consensus on Catheter Ablation of Ventricular Arrhythmias: developed in a partnership with the European Heart Rhythm Association (EHRA), a Registered Branch of the European Society of Cardiology (ESC), and the Heart Rhythm Society (HRS); in collaboration with the American College of Cardiology (ACC) and the American Heart Association (AHA) Europace. 2009;11:771–817. doi: 10.1093/europace/eup098. [DOI] [PubMed] [Google Scholar]
- 7.Sacher F, Roberts-Thomson K, Maury P, Tedrow U, Nault I, Steven D, Hocini M, Koplan B, Leroux L, Derval N, Seiler J, Wright MJ, Epstein L, Haissaguerre M, Jais P, Stevenson WG. Epicardial ventricular tachycardia ablation a multicenter safety study. J Am Coll Cardiol. 2010;55:2366–2372. doi: 10.1016/j.jacc.2009.10.084. [DOI] [PubMed] [Google Scholar]
- 8.Abrahams P, Boon J, Spratt J. McMinn’s Clinical Atlas of Human Anatomy. 6. Mosby-Elsevier; 2008. pp. 188–189. [Google Scholar]
- 9.Loukas M, Walters A, Boon JM, Welch TP, Meiring JH, Abrahams PH. Pericardiocentesis: A clinical anatomy review. Clinical anatomy. 2012 doi: 10.1002/ca.22032. [DOI] [PubMed] [Google Scholar]
- 10.D’Avila A, Scanavacca M, Sosa E, Ruskin JN, Reddy VY. Pericardial anatomy for the interventional electrophysiologist. Journal of cardiovascular electrophysiology. 2003;14:422–430. doi: 10.1046/j.1540-8167.2003.02487.x. [DOI] [PubMed] [Google Scholar]
- 11.Lachman N, Syed FF, Habib A, Kapa S, Bisco SE, Venkatachalam KL, Asirvatham SJ. Correlative anatomy for the electrophysiologist, Part I: the pericardial space, oblique sinus, transverse sinus. Journal of cardiovascular electrophysiology. 2010;21:1421–1426. doi: 10.1111/j.1540-8167.2010.01872.x. [DOI] [PubMed] [Google Scholar]
- 12.Ernst S, Sanchez-Quintana D, Ho SY. Anatomy of the pericardial space and mediastinum:relevance to epicardial mapping and ablation. In: Shivkumar K, Boyle NG, editors. Cardiac Electrophysiology Clinics: Epicardial Interventions in Electrophysiology. Philadelphia, PA: Saunders; 2009. pp. 1–8. [DOI] [PubMed] [Google Scholar]
- 13.Lachman N, Syed FF, Habib A, Kapa S, Bisco SE, Venkatachalam KL, Asirvatham SJ. Correlative anatomy for the electrophysiologist, part II: cardiac ganglia, phrenic nerve, coronary venous system. Journal of cardiovascular electrophysiology. 2011;22:104–110. doi: 10.1111/j.1540-8167.2010.01882.x. [DOI] [PubMed] [Google Scholar]
- 14.Drury NE, De Silva RJ, Hall RM, Large SR. Congenital defects of the pericardium. The Annals of thoracic surgery. 2007;83:1552–1553. doi: 10.1016/j.athoracsur.2006.10.063. [DOI] [PubMed] [Google Scholar]
- 15.O’Leary SM, Williams PL, Williams MP, Edwards AJ, Roobottom CA, Morgan-Hughes GJ, Manghat NE. Imaging the pericardium: appearances on ECG-gated 64-detector row cardiac computed tomography. The British journal of radiology. 2010;83:194–205. doi: 10.1259/bjr/55699491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broderick LS, Brooks GN, Kuhlman JE. Anatomic pitfalls of the heart and pericardium. Radiographics : a review publication of the Radiological Society of North America, Inc. 2005;25:441–453. doi: 10.1148/rg.252045075. [DOI] [PubMed] [Google Scholar]
- 17.Choe YH, Im JG, Park JH, Han MC, Kim CW. The anatomy of the pericardial space: a study in cadavers and patients. AJR. American journal of roentgenology. 1987;149:693–697. doi: 10.2214/ajr.149.4.693. [DOI] [PubMed] [Google Scholar]
- 18.Fritscher-Ravens A, Ganbari A, Mosse CA, Swain P, Koehler P, Patel K. Transesophageal endoscopic ultrasound-guided access to the heart. Endoscopy. 2007;39:385–389. doi: 10.1055/s-2007-966440. [DOI] [PubMed] [Google Scholar]
- 19.Verrier RL, Waxman S, Lovett EG, Moreno R. Transatrial access to the normal pericardial space: a novel approach for diagnostic sampling, pericardiocentesis, and therapeutic interventions. Circulation. 1998;98:2331–2333. doi: 10.1161/01.cir.98.21.2331. [DOI] [PubMed] [Google Scholar]
- 20.Scanavacca MI, Venancio AC, Pisani CF, Lara S, Hachul D, Darrieux F, Hardy C, Paola E, Aiello VD, Mahapatra S, Sosa E. Percutaneous transatrial access to the pericardial space for epicardial mapping and ablation. Circulationw Arrhythmia and electrophysiology. 2011;4:331–336. doi: 10.1161/CIRCEP.110.960799. [DOI] [PubMed] [Google Scholar]
- 21.Ceron L, Manzato M, Mazzaro F, Bellavere F. A new diagnostic and therapeutic approach to pericardial effusion: transbronchial needle aspiration. Chest. 2003;123:1753–1758. doi: 10.1378/chest.123.5.1753. [DOI] [PubMed] [Google Scholar]
- 22.Soejima K, Couper G, Cooper JM, Sapp JL, Epstein LM, Stevenson WG. Subxiphoid surgical approach for epicardial catheter-based mapping and ablation in patients with prior cardiac surgery or difficult pericardial access. Circulation. 2004;110:1197–1201. doi: 10.1161/01.CIR.0000140725.42845.90. [DOI] [PubMed] [Google Scholar]
- 23.Michowitz Y, Mathuria N, Tung R, Esmailian F, Kwon M, Nakahara S, Bourke T, Boyle NG, Mahajan A, Shivkumar K. Hybrid procedures for epicardial catheter ablation of ventricular tachycardia: value of surgical access. Heart Rhythm. 2010;7:1635–1643. doi: 10.1016/j.hrthm.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Sosa E, Scanavacca M, D’Avila A, Antonio J, Ramires F. Nonsurgical transthoracic epicardial approach in patients with ventricular tachycardia and previous cardiac surgery. J Interv Card Electrophysiol. 2004;10:281–288. doi: 10.1023/B:JICE.0000026925.41543.7c. [DOI] [PubMed] [Google Scholar]
- 25.Sosa E, Scanavacca M, D’Avila A, Piccioni J, Sanchez O, Velarde JL, Silva M, Reolao B. Endocardial and epicardial ablation guided by nonsurgical transthoracic epicardial mapping to treat recurrent ventricular tachycardia. Journal of cardiovascular electrophysiology. 1998;9:229–239. doi: 10.1111/j.1540-8167.1998.tb00907.x. [DOI] [PubMed] [Google Scholar]
- 26.Sosa E, Scanavacca M, d’Avila A, Oliveira F, Ramires JA. Nonsurgical transthoracic epicardial catheter ablation to treat recurrent ventricular tachycardia occurring late after myocardial infarction. Journal of the American College of Cardiology. 2000;35:1442–1449. doi: 10.1016/s0735-1097(00)00606-9. [DOI] [PubMed] [Google Scholar]
- 27.Schweikert RA, Saliba WI, Tomassoni G, Marrouche NF, Cole CR, Dresing TJ, Tchou PJ, Bash D, Beheiry S, Lam C, Kanagaratnam L, Natale A. Percutaneous pericardial instrumentation for endo-epicardial mapping of previously failed ablations. Circulation. 2003;108:1329–1335. doi: 10.1161/01.CIR.0000087407.53326.31. [DOI] [PubMed] [Google Scholar]
- 28.Sarabanda AV, Sosa E, Simoes MV, Figueiredo GL, Pintya AO, Marin-Neto JA. Ventricular tachycardia in Chagas’ disease: a comparison of clinical, angiographic, electrophysiologic and myocardial perfusion disturbances between patients presenting with either sustained or nonsustained forms. International journal of cardiology. 2005;102:9–19. doi: 10.1016/j.ijcard.2004.03.087. [DOI] [PubMed] [Google Scholar]
- 29.Cesario DA, Vaseghi M, Boyle NG, Fishbein MC, Valderrabano M, Narasimhan C, Wiener I, Shivkumar K. Value of high-density endocardial and epicardial mapping for catheter ablation of hemodynamically unstable ventricular tachycardia. Heart Rhythm. 2006;3:1–10. doi: 10.1016/j.hrthm.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 30.Daniels DV, Lu YY, Morton JB, Santucci PA, Akar JG, Green A, Wilber DJ. Idiopathic epicardial left ventricular tachycardia originating remote from the sinus of Valsalva: electrophysiological characteristics, catheter ablation, and identification from the 12-lead electrocardiogram. Circulation. 2006;113:1659–1666. doi: 10.1161/CIRCULATIONAHA.105.611640. [DOI] [PubMed] [Google Scholar]
- 31.Aryana A, d’Avila A, Heist EK, Mela T, Singh JP, Ruskin JN, Reddy VY. Remote magnetic navigation to guide endocardial and epicardial catheter mapping of scar-related ventricular tachycardia. Circulation. 2007;115:1191–1200. doi: 10.1161/CIRCULATIONAHA.106.672162. [DOI] [PubMed] [Google Scholar]
- 32.Garcia FC, Bazan V, Zado ES, Ren JF, Marchlinski FE. Epicardial substrate and outcome with epicardial ablation of ventricular tachycardia in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation. 2009;120:366–375. doi: 10.1161/CIRCULATIONAHA.108.834903. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt B, Chun KR, Baensch D, Antz M, Koektuerk B, Tilz RR, Metzner A, Ouyang F, Kuck KH. Catheter ablation for ventricular tachycardia after failed endocardial ablation: epicardial substrate or inappropriate endocardial ablation? Heart Rhythm. 2010;7:1746–1752. doi: 10.1016/j.hrthm.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 34.Nakahara S, Tung R, Ramirez RJ, Michowitz Y, Vaseghi M, Buch E, Gima J, Wiener I, Mahajan A, Boyle NG, Shivkumar K. Characterization of the arrhythmogenic substrate in ischemic and nonischemic cardiomyopathy implications for catheter ablation of hemodynamically unstable ventricular tachycardia. Journal of the American College of Cardiology. 2010;55:2355–2365. doi: 10.1016/j.jacc.2010.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nademanee K, Veerakul G, Chandanamattha P, Chaothawee L, Ariyachaipanich A, Jirasirirojanakorn K, Likittanasombat K, Bhuripanyo K, Ngarmukos T. Prevention of ventricular fibrillation episodes in Brugada syndrome by catheter ablation over the anterior right ventricular outflow tract epicardium. Circulation. 2011;123:1270–1279. doi: 10.1161/CIRCULATIONAHA.110.972612. [DOI] [PubMed] [Google Scholar]
- 36.Bai R, Di Biase L, Shivkumar K, Mohanty P, Tung R, Santangeli P, Saenz LC, Vacca M, Verma A, Khaykin Y, Mohanty S, Burkhardt JD, Hongo R, Beheiry S, Dello Russo A, Casella M, Pelargonio G, Santarelli P, Sanchez J, Tondo C, Natale A. Ablation of ventricular arrhythmias in arrhythmogenic right ventricular dysplasia/cardiomyopathy: arrhythmia-free survival after endo-epicardial substrate based mapping and ablation. Circulation. Arrhythmia and electrophysiology. 2011;4:478–485. doi: 10.1161/CIRCEP.111.963066. [DOI] [PubMed] [Google Scholar]
- 37.Della Bella P, Brugada J, Zeppenfeld K, Merino J, Neuzil P, Maury P, Maccabelli G, Vergara P, Baratto F, Berruezo A, Wijnmaalen AP. Epicardial ablation for ventricular tachycardia: a European multicenter study. Circulation. Arrhythmia and electrophysiology. 2011;4:653–659. doi: 10.1161/CIRCEP.111.962217. [DOI] [PubMed] [Google Scholar]
- 38.Dello Russo A, Casella M, Pieroni M, Pelargonio G, Bartoletti S, Santangeli P, Zucchetti M, Innocenti E, Di Biase L, Carbucicchio C, Bellocci F, Fiorentini C, Natale A, Tondo C. Drug-Refractory Ventricular Tachycardias Following Myocarditis: Endocardial and Epicardial Radiofrequency Catheter Ablation. Circulation Arrhythmia and electrophysiology. 2012 doi: 10.1161/CIRCEP.111.965012. [DOI] [PubMed] [Google Scholar]
- 39.Sosa E, Scanavacca M, D’Avila A, Bellotti G, Pilleggi F. Radiofrequency catheter ablation of ventricular tachycardia guided by nonsurgical epicardial mapping in chronic Chagasic heart disease. Pacing and clinical electrophysiology : PACE. 1999;22:128–130. doi: 10.1111/j.1540-8159.1999.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 40.Berruezo A, Mont L, Nava S, Chueca E, Bartholomay E, Brugada J. Electrocardiographic recognition of the epicardial origin of ventricular tachycardias. Circulation. 2004;109:1842–1847. doi: 10.1161/01.CIR.0000125525.04081.4B. [DOI] [PubMed] [Google Scholar]
- 41.Martinek M, Stevenson WG, Inada K, Tokuda M, Tedrow UB. QRS characteristics fail to reliably identify ventricular tachycardias that require epicardial ablation in ischemic heart disease. Journal of cardiovascular electrophysiology. 2012;23:188–193. doi: 10.1111/j.1540-8167.2011.02179.x. [DOI] [PubMed] [Google Scholar]
- 42.Bazan V, Bala R, Garcia FC, Sussman JS, Gerstenfeld EP, Dixit S, Callans DJ, Zado E, Marchlinski FE. Twelve-lead ECG features to identify ventricular tachycardia arising from the epicardial right ventricle. Heart Rhythm. 2006;3:1132–1139. doi: 10.1016/j.hrthm.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 43.Bazan V, Gerstenfeld EP, Garcia FC, Bala R, Rivas N, Dixit S, Zado E, Callans DJ, Marchlinski FE. Site-specific twelve-lead ECG features to identify an epicardial origin for left ventricular tachycardia in the absence of myocardial infarction. Heart Rhythm. 2007;4:1403–1410. doi: 10.1016/j.hrthm.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 44.Valles E, Bazan V, Marchlinski FE. ECG criteria to identify epicardial ventricular tachycardia in nonischemic cardiomyopathy. Circulation. Arrhythmia and electrophysiology. 2010;3:63–71. doi: 10.1161/CIRCEP.109.859942. [DOI] [PubMed] [Google Scholar]
- 45.Michowitz Y, Belhassen B. Electrocardiographic recognition od Epicardial Arrhythmias. In: Shivkumar K, Boyle NG, editors. Cardiac Electrophysiology Clinics: Epicardial Interventions in Cardiac Electrophysiology. Vol. 2. Vol. 2100. Philadelphia, PA: Saunders; pp. 25–33. [DOI] [PubMed] [Google Scholar]
- 46.Scanavacca M, Sosa E, d’Avila A, De Lourdes Higuchi M. Radiofrequency ablation of sustained ventricular tachycardia related to the mitral isthmus in Chagas’ disease. Pacing and clinical electrophysiology : PACE. 2002;25:368–371. doi: 10.1046/j.1460-9592.2002.00368.x. [DOI] [PubMed] [Google Scholar]
- 47.Scanavacca M, Sosa E. Epicardial Ablation of Ventricular Tachycardia in Chagas Heart Disease. In: Shivkumar K, Boyle NG, editors. Cardiac Electrophysiology Clinics: Epicardial Interventions in Electrophysiology. Vol. 2. Philadelphia, PA: Saunders; 2010. pp. 55–67. [DOI] [PubMed] [Google Scholar]
- 48.Nakahara S, Tung R, Ramirez RJ, Gima J, Wiener I, Mahajan A, Boyle NG, Shivkumar K. Distribution of late potentials within infarct scars assessed by ultra high-density mapping. Heart Rhythm. 2010;7:1817–1824. doi: 10.1016/j.hrthm.2010.07.032. [DOI] [PubMed] [Google Scholar]
- 49.Nakahara S, Tung R, Ramirez RJ, Michowitz Y, Vaseghi M, Buch E, Gima J, Wiener I, Mahajan A, Boyle NG, Shivkumar K. Characterization of the arrhythmogenic substrate in ischemic and nonischemic cardiomyopathy implications for catheter ablation of hemodynamically unstable ventricular tachycardia. J Am Coll Cardiol. 2010;55:2355–2365. doi: 10.1016/j.jacc.2010.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tung R, Nakahara S, Maccabelli G, Buch E, Wiener I, Boyle NG, Carbucicchio C, Bella PD, Shivkumar K. Ultra high-density multipolar mapping with double ventricular access: a novel technique for ablation of ventricular tachycardia. J Cardiovasc Electrophysiol. 2011;22:49–56. doi: 10.1111/j.1540-8167.2010.01859.x. [DOI] [PubMed] [Google Scholar]
- 51.Hsia HH, Callans DJ, Marchlinski FE. Characterization of endocardial electrophysiological substrate in patients with nonischemic cardiomyopathy and monomorphic ventricular tachycardia. Circulation. 2003;108:704–710. doi: 10.1161/01.CIR.0000083725.72693.EA. [DOI] [PubMed] [Google Scholar]
- 52.Soejima K, Stevenson WG, Sapp JL, Selwyn AP, Couper G, Epstein LM. Endocardial and epicardial radiofrequency ablation of ventricular tachycardia associated with dilated cardiomyopathy: the importance of low-voltage scars. Journal of the American College of Cardiology. 2004;43:1834–1842. doi: 10.1016/j.jacc.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 53.Cano O, Hutchinson M, Lin D, Garcia F, Zado E, Bala R, Riley M, Cooper J, Dixit S, Gerstenfeld E, Callans D, Marchlinski FE. Electroanatomic substrate and ablation outcome for suspected epicardial ventricular tachycardia in left ventricular nonischemic cardiomyopathy. Journal of the American College of Cardiology. 2009;54:799–808. doi: 10.1016/j.jacc.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 54.Graner M, Lommi J, Kupari M, Raisanen-Sokolowski A, Toivonen L. Multiple forms of sustained monomorphic ventricular tachycardia as common presentation in giant-cell myocarditis. Heart. 2007;93:119–121. doi: 10.1136/hrt.2005.079053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hama Y, Funabashi N, Ueda M, Kanaeda T, Uehara M, Nakamura K, Murayama T, Mikami Y, Takaoka H, Kawakubo M, Lee K, Takano H, Komuro I. Images in cardiovascular medicine. Right-sided heart wall thickening and delayed enhancement caused by chronic active myocarditis complicated by sustained monomorphic ventricular tachycardia. Circulation. 2009;119:e200–203. doi: 10.1161/CIRCULATIONAHA.108.788380. [DOI] [PubMed] [Google Scholar]
- 56.De Cobelli F, Pieroni M, Esposito A, Chimenti C, Belloni E, Mellone R, Canu T, Perseghin G, Gaudio C, Maseri A, Frustaci A, Del Maschio A. Delayed gadolinium-enhanced cardiac magnetic resonance in patients with chronic myocarditis presenting with heart failure or recurrent arrhythmias. Journal of the American College of Cardiology. 2006;47:1649–1654. doi: 10.1016/j.jacc.2005.11.067. [DOI] [PubMed] [Google Scholar]
- 57.Uusimaa P, Ylitalo K, Anttonen O, Kerola T, Virtanen V, Paakko E, Raatikainen P. Ventricular tachyarrhythmia as a primary presentation of sarcoidosis. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2008;10:760–766. doi: 10.1093/europace/eun110. [DOI] [PubMed] [Google Scholar]
- 58.Koplan BA, Soejima K, Baughman K, Epstein LM, Stevenson WG. Refractory ventricular tachycardia secondary to cardiac sarcoid: electrophysiologic characteristics, mapping, and ablation. Heart Rhythm. 2006;3:924–929. doi: 10.1016/j.hrthm.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 59.Jefic D, Joel B, Good E, Morady F, Rosman H, Knight B, Bogun F. Role of radiofrequency catheter ablation of ventricular tachycardia in cardiac sarcoidosis: report from a multicenter registry. Heart Rhythm. 2009;6:189–195. doi: 10.1016/j.hrthm.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 60.Marcus FI, Fontaine GH, Guiraudon G, Frank R, Laurenceau JL, Malergue C, Grosgogeat Y. Right ventricular dysplasia: a report of 24 adult cases. Circulation. 1982;65:384–398. doi: 10.1161/01.cir.65.2.384. [DOI] [PubMed] [Google Scholar]
- 61.Guiraudon GM, Klein GJ, Gulamhusein SS, Painvin GA, Del Campo C, Gonzales JC, Ko PT. Total disconnection of the right ventricular free wall: surgical treatment of right ventricular tachycardia associated with right ventricular dysplasia. Circulation. 1983;67:463–470. doi: 10.1161/01.cir.67.2.463. [DOI] [PubMed] [Google Scholar]
- 62.Marchlinski FE, Zado E, Dixit S, Gerstenfeld E, Callans DJ, Hsia H, Lin D, Nayak H, Russo A, Pulliam W. Electroanatomic substrate and outcome of catheter ablative therapy for ventricular tachycardia in setting of right ventricular cardiomyopathy. Circulation. 2004;110:2293–2298. doi: 10.1161/01.CIR.0000145154.02436.90. [DOI] [PubMed] [Google Scholar]
- 63.Berruezo A, Fernandez-Armenta J, Mont L, Zeljko H, Andreu D, Herczku C, Boussy T, Tolosana JM, Arbelo E, Brugada J. Combined endocardial and epicardial catheter ablation in arrhythmogenic right ventricular dysplasia incorporating scar dechanneling technique. Circulation. Arrhythmia and electrophysiology. 2012;5:111–121. doi: 10.1161/CIRCEP.110.960740. [DOI] [PubMed] [Google Scholar]
- 64.Nagase S, Kusano KF, Morita H, Nishii N, Banba K, Watanabe A, Hiramatsu S, Nakamura K, Sakuragi S, Ohe T. Longer repolarization in the epicardium at the right ventricular outflow tract causes type 1 electrocardiogram in patients with Brugada syndrome. Journal of the American College of Cardiology. 2008;51:1154–1161. doi: 10.1016/j.jacc.2007.10.059. [DOI] [PubMed] [Google Scholar]
- 65.Josephson ME, Harken AH, Horowitz LN. Endocardial excision: a new surgical technique for the treatment of recurrent ventricular tachycardia. Circulation. 1979;60:1430–1439. doi: 10.1161/01.cir.60.7.1430. [DOI] [PubMed] [Google Scholar]
- 66.Mathuria NS, Vaseghi M, Buch E, Shivkumar K. Successful ablation of an epicardial ventricular tachycardia using a surgical ablation tool. Circulation. Arrhythmia and electrophysiology. 2011;4:e84–86. doi: 10.1161/CIRCEP.111.965467. [DOI] [PubMed] [Google Scholar]
- 67.Anter E, Hutchinson MD, Deo R, Haqqani HM, Callans DJ, Gerstenfeld EP, Garcia FC, Bala R, Lin D, Riley MP, Litt HI, Woo JY, Acker MA, Szeto WY, Zado ES, Marchlinski FE, Dixit S. Surgical ablation of refractory ventricular tachycardia in patients with nonischemic cardiomyopathy. Circulation. Arrhythmia and electrophysiology. 2011;4:494–500. doi: 10.1161/CIRCEP.111.962555. [DOI] [PubMed] [Google Scholar]
- 68.Stevenson WG, Couper GS. A surgical option for ventricular tachycardia caused by nonischemic cardiomyopathy. Circ Arrhythm Electrophysiol. 2011;4:429–431. doi: 10.1161/CIRCEP.111.964189. [DOI] [PubMed] [Google Scholar]
- 69.Tedrow U, Stevenson WG. Strategies for epicardial mapping and ablation of ventricular tachycardia. Journal of cardiovascular electrophysiology. 2009;20:710–713. doi: 10.1111/j.1540-8167.2008.01427.x. [DOI] [PubMed] [Google Scholar]
- 70.Katritsis D, Giazitzoglou E, Korovesis S, Paxinos G, Anagnostopoulos CE, Camm AJ. Epicardial foci of atrial arrhythmias apparently originating in the left pulmonary veins. Journal of cardiovascular electrophysiology. 2002;13:319–323. doi: 10.1046/j.1540-8167.2002.00319.x. [DOI] [PubMed] [Google Scholar]
- 71.Valderrabano M, Cesario DA, Ji S, Shannon K, Wiener I, Swerdlow CD, Oral H, Morady F, Shivkumar K. Percutaneous epicardial mapping during ablation of difficult accessory pathways as an alternative to cardiac surgery. Heart Rhythm. 2004;1:311–316. doi: 10.1016/j.hrthm.2004.03.073. [DOI] [PubMed] [Google Scholar]
- 72.Scanavacca M, Pisani CF, Hachul D, Lara S, Hardy C, Darrieux F, Trombetta I, Negrao CE, Sosa E. Selective atrial vagal denervation guided by evoked vagal reflex to treat patients with paroxysmal atrial fibrillation. Circulation. 2006;114:876–885. doi: 10.1161/CIRCULATIONAHA.106.633560. [DOI] [PubMed] [Google Scholar]
- 73.Pak HN, Hwang C, Lim HE, Kim JS, Kim YH. Hybrid epicardial and endocardial ablation of persistent or permanent atrial fibrillation: a new approach for difficult cases. Journal of cardiovascular electrophysiology. 2007;18:917–923. doi: 10.1111/j.1540-8167.2007.00882.x. [DOI] [PubMed] [Google Scholar]
- 74.Phillips KP, Natale A, Sterba R, Saliba WI, Burkhardt JD, Wazni O, Liberman L, Schweikert RA. Percutaneous pericardial instrumentation for catheter ablation of focal atrial tachycardias arising from the left atrial appendage. Journal of cardiovascular electrophysiology. 2008;19:430–433. doi: 10.1111/j.1540-8167.2007.01028.x. [DOI] [PubMed] [Google Scholar]
- 75.Haissaguerre M, Gaita F, Fischer B, Egloff P, Lemetayer P, Warin JF. Radiofrequency catheter ablation of left lateral accessory pathways via the coronary sinus. Circulation. 1992;86:1464–1468. doi: 10.1161/01.cir.86.5.1464. [DOI] [PubMed] [Google Scholar]
- 76.Kleinman D, Winters SL. Successful catheter ablation of an inferoseptal accessory pathway within the coronary sinus in a patient with a previously unsuccessful attempt at surgical interruption. Coronary sinus ablation for Wolff-Parkinson-White syndrome. Journal of electrocardiology. 1996;29:55–60. doi: 10.1016/s0022-0736(96)80113-0. [DOI] [PubMed] [Google Scholar]
- 77.Beukema WP, Van Dessel PF, Van Hemel NM, Kingma JH. Radiofrequency catheter ablation of accessory pathways associated with a coronary sinus diverticulum. European heart journal. 1994;15:1415–1418. doi: 10.1093/oxfordjournals.eurheartj.a060404. [DOI] [PubMed] [Google Scholar]
- 78.Sun Y, Arruda M, Otomo K, Beckman K, Nakagawa H, Calame J, Po S, Spector P, Lustgarten D, Herring L, Lazzara R, Jackman W. Coronary sinus-ventricular accessory connections producing posteroseptal and left posterior accessory pathways: incidence and electrophysiological identification. Circulation. 2002;106:1362–1367. doi: 10.1161/01.cir.0000028464.12047.a6. [DOI] [PubMed] [Google Scholar]
- 79.Morady F, Strickberger A, Man KC, Daoud E, Niebauer M, Goyal R, Harvey M, Bogun F. Reasons for prolonged or failed attempts at radiofrequency catheter ablation of accessory pathways. Journal of the American College of Cardiology. 1996;27:683–689. doi: 10.1016/0735-1097(95)00493-9. [DOI] [PubMed] [Google Scholar]
- 80.Sacher F, Wright M, Tedrow UB, O’Neill MD, Jais P, Hocini M, Macdonald R, Davies DW, Kanagaratnam P, Derval N, Epstein L, Peters NS, Stevenson WG, Haissaguerre M. Wolff-Parkinson-White ablation after a prior failure: a 7-year multicentre experience. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2010;12:835–841. doi: 10.1093/europace/euq050. [DOI] [PubMed] [Google Scholar]
- 81.Koplan BA, Parkash R, Couper G, Stevenson WG. Combined epicardial-endocardial approach to ablation of inappropriate sinus tachycardia. Journal of cardiovascular electrophysiology. 2004;15:237–240. doi: 10.1046/j.1540-8167.2004.03370.x. [DOI] [PubMed] [Google Scholar]
- 82.Shivkumar K. Percutaneous epicardial ablation of atrial fibrillation. Heart Rhythm. 2008;5:152–154. doi: 10.1016/j.hrthm.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 83.Hwang C, Wu TJ, Doshi RN, Peter CT, Chen PS. Vein of marshall cannulation for the analysis of electrical activity in patients with focal atrial fibrillation. Circulation. 2000;101:1503–1505. doi: 10.1161/01.cir.101.13.1503. [DOI] [PubMed] [Google Scholar]
- 84.Kowalski M, Grimes MM, Perez FJ, Kenigsberg DN, Koneru J, Kasirajan V, Wood MA, Ellenbogen KA. Histopathologic characterization of chronic radiofrequency ablation lesions for pulmonary vein isolation. Journal of the American College of Cardiology. 2012;59:930–938. doi: 10.1016/j.jacc.2011.09.076. [DOI] [PubMed] [Google Scholar]
- 85.Choi JI, Pak HN, Kim YH. Hybrid epicardial and endocardial catheter ablation in a patient with atrial fibrillation and suspicious left atrial thrombus. Circulation journal : official journal of the Japanese Circulation Society. 2009;73:384–387. doi: 10.1253/circj.cj-08-0276. [DOI] [PubMed] [Google Scholar]
- 86.Reddy VY, Neuzil P, D’Avila A, Ruskin JN. Isolating the posterior left atrium and pulmonary veins with a “box” lesion set: use of epicardial ablation to complete electrical isolation. Journal of cardiovascular electrophysiology. 2008;19:326–329. doi: 10.1111/j.1540-8167.2007.00944.x. [DOI] [PubMed] [Google Scholar]
- 87.Sosa E, Scanavacca M. Epicardial Approach to Catheter Ablation of Ventricular Tachycardia. In: Wood M, Huang S, editors. Catheter Ablation of Cardiac Arrhythmias, 2e. 2. Philadelphia, PA: Elsevier; 2011. pp. 560–573. [Google Scholar]
- 88.Sosa E, Scanavacca M, d’Avila A. Transthoracic epicardial catheter ablation to treat recurrent ventricular tachycardia. Current cardiology reports. 2001;3:451–458. doi: 10.1007/s11886-001-0066-1. [DOI] [PubMed] [Google Scholar]
- 89.Koruth JS, Aryana A, Dukkipati SR, Pak HN, Kim YH, Sosa EA, Scanavacca M, Mahapatra S, Ailawadi G, Reddy VY, d’Avila A. Unusual complications of percutaneous epicardial access and epicardial mapping and ablation of cardiac arrhythmias. Circulation. Arrhythmia and electrophysiology. 2011;4:882–888. doi: 10.1161/CIRCEP.111.965731. [DOI] [PubMed] [Google Scholar]
- 90.Mathuria N, Buch E, Shivkumar K. Pleuropericardial fistula formation after prior epicardial catheter ablation for ventricular tachycardia. Circulation. Arrhythmia and electrophysiology. 2012;5:e18–19. doi: 10.1161/CIRCEP.111.968420. [DOI] [PubMed] [Google Scholar]
- 91.Tschabrunn CM, Haqqani HM, Zado ES, Marchlinski FE. Repeat Percutaneous Epicardial Mapping and Ablation of Ventricular Tachycardia:: Safety and Outcome. Journal of cardiovascular electrophysiology. 2012 doi: 10.1111/j.1540-8167.2011.02286.x. [DOI] [PubMed] [Google Scholar]
- 92.Javaheri A, Glassberg HL, Acker MA, Callans DJ, Goldberg LR. Constrictive pericarditis presenting as a late complication of epicardial ventricular tachycardia ablation. Circulation. Heart failure. 2012;5:e22–23. doi: 10.1161/CIRCHEARTFAILURE.111.965236. [DOI] [PubMed] [Google Scholar]
- 93.D’Avila A, Neuzil P, Thiagalingam A, Gutierrez P, Aleong R, Ruskin JN, Reddy VY. Experimental efficacy of pericardial instillation of anti-inflammatory agents during percutaneous epicardial catheter ablation to prevent postprocedure pericarditis. Journal of cardiovascular electrophysiology. 2007;18:1178–1183. doi: 10.1111/j.1540-8167.2007.00945.x. [DOI] [PubMed] [Google Scholar]
- 94.Buch E, Vaseghi M, Cesario DA, Shivkumar K. A novel method for preventing phrenic nerve injury during catheter ablation. Heart Rhythm. 2007;4:95–98. doi: 10.1016/j.hrthm.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fan R, Cano O, Ho SY, Bala R, Callans DJ, Dixit S, Garcia F, Gerstenfeld EP, Hutchinson M, Lin D, Riley M, Marchlinski FE. Characterization of the phrenic nerve course within the epicardial substrate of patients with nonischemic cardiomyopathy and ventricular tachycardia. Heart Rhythm. 2009;6:59–64. doi: 10.1016/j.hrthm.2008.09.033. [DOI] [PubMed] [Google Scholar]
- 96.Di Biase L, Burkhardt JD, Pelargonio G, Dello Russo A, Casella M, Santarelli P, Horton R, Sanchez J, Gallinghouse JG, Al-Ahmad A, Wang P, Cummings JE, Schweikert RA, Natale A. Prevention of phrenic nerve injury during epicardial ablation: comparison of methods for separating the phrenic nerve from the epicardial surface. Heart Rhythm. 2009;6:957–961. doi: 10.1016/j.hrthm.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 97.Buch E, Nakahara S, Shivkumar K. Intra-pericardial balloon retraction of the left atrium: a novel method to prevent esophageal injury during catheter ablation. Heart Rhythm. 2008;5:1473–1475. doi: 10.1016/j.hrthm.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nakahara S, Ramirez RJ, Buch E, Michowitz Y, Vaseghi M, de Diego C, Boyle NG, Mahajan A, Shivkumar K. Intrapericardial balloon placement for prevention of collateral injury during catheter ablation of the left atrium in a porcine model. Heart Rhythm. 2010;7:81–87. doi: 10.1016/j.hrthm.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee JC, Steven D, Roberts-Thomson KC, Raymond JM, Stevenson WG, Tedrow UB. Atrial tachycardias adjacent to the phrenic nerve: recognition, potential problems, and solutions. Heart Rhythm. 2009;6:1186–1191. doi: 10.1016/j.hrthm.2009.03.056. [DOI] [PubMed] [Google Scholar]
- 100.Hammill SC. Epicardial ablation: reducing the risks. Journal of cardiovascular electrophysiology. 2006;17:550–552. doi: 10.1111/j.1540-8167.2006.00459.x. [DOI] [PubMed] [Google Scholar]
- 101.D’Avila A, Gutierrez P, Scanavacca M, Reddy V, Lustgarten DL, Sosa E, Ramires JA. Effects of radiofrequency pulses delivered in the vicinity of the coronary arteries: implications for nonsurgical transthoracic epicardial catheter ablation to treat ventricular tachycardia. Pacing and clinical electrophysiology : PACE. 2002;25:1488–1495. doi: 10.1046/j.1460-9592.2002.01488.x. [DOI] [PubMed] [Google Scholar]
- 102.Thyer IA, Kovoor P, Barry MA, Pouliopoulos J, Ross DL, Thiagalingam A. Protection of the coronary arteries during epicardial radiofrequency ablation with intracoronary chilled saline irrigation: assessment in an in vitro model. Journal of cardiovascular electrophysiology. 2006;17:544–549. doi: 10.1111/j.1540-8167.2006.00417.x. [DOI] [PubMed] [Google Scholar]
- 103.Viles-Gonzalez JF, de Castro Miranda R, Scanavacca M, Sosa E, d’Avila A. Acute and chronic effects of epicardial radiofrequency applications delivered on epicardial coronary arteries. Circulation. Arrhythmia and electrophysiology. 2011;4:526–531. doi: 10.1161/CIRCEP.110.961508. [DOI] [PubMed] [Google Scholar]
- 104.Mahapatra S, Tucker-Schwartz J, Wiggins D, Gillies GT, Mason PK, McDaniel G, Lapar DJ, Stemland C, Sosa E, Ferguson JD, Bunch TJ, Ailawadi G, Scanavacca M. Pressure frequency characteristics of the pericardial space and thorax during subxiphoid access for epicardial ventricular tachycardia ablation. Heart Rhythm. 2010;7:604–609. doi: 10.1016/j.hrthm.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pollak PM, Mahapatra S, Kanwal JK, Gillies GT. Novel pericardial access device: design features and in vitro evaluation. Journal of medical engineering & technology. 2011;35:179–184. doi: 10.3109/03091902.2011.558171. [DOI] [PubMed] [Google Scholar]
- 106.D’Avila A, Houghtaling C, Gutierrez P, Vragovic O, Ruskin JN, Josephson ME, Reddy VY. Catheter ablation of ventricular epicardial tissue: a comparison of standard and cooled-tip radiofrequency energy. Circulation. 2004;109:2363–2369. doi: 10.1161/01.CIR.0000128039.87485.0B. [DOI] [PubMed] [Google Scholar]
- 107.Di Biase L, Saliba WI, Natale A. Successful ablation of epicardial arrhythmias with cryoenergy after failed attempts with radiofrequency energy. Heart Rhythm. 2009;6:109–112. doi: 10.1016/j.hrthm.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 108.Lustgarten DL, Bell S, Hardin N, Calame J, Spector PS. Safety and efficacy of epicardial cryoablation in a canine model. Heart Rhythm. 2005;2:82–90. doi: 10.1016/j.hrthm.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 109.D’Avila A, Aryana A, Thiagalingam A, Holmvang G, Schmidt E, Gutierrez P, Ruskin JN, Reddy VY. Focal and linear endocardial and epicardial catheter-based cryoablation of normal and infarcted ventricular tissue. Pacing Clin Electrophysiol. 2008;31:1322–1331. doi: 10.1111/j.1540-8159.2008.01184.x. [DOI] [PubMed] [Google Scholar]
- 110.Ghia K, Haines D. Energy Sources for Ablation in the Pericardial space. In: Shivkumar K, Boyle NG, editors. Cardiac Electrophysiology Clinics: Epicardial Interventions in Electrophysiology. Vol. 2. Philadelphia, PA: Saunders; 2010. pp. 45–54. [DOI] [PubMed] [Google Scholar]
- 111.Tung R, Nakahara S, Ramirez R, Lai C, Fishbein MC, Shivkumar K. Distinguishing epicardial fat from scar: analysis of electrograms using high-density electroanatomic mapping in a novel porcine infarct model. Heart Rhythm. 2010;7:389–395. doi: 10.1016/j.hrthm.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Al-Saady NM, Obel OA, Camm AJ. Left atrial appendage: structure, function, and role in thromboembolism. Heart. 1999;82:547–554. doi: 10.1136/hrt.82.5.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hanna IR, Kolm P, Martin R, Reisman M, Gray W, Block PC. Left atrial structure and function after percutaneous left atrial appendage transcatheter occlusion (PLAATO): six-month echocardiographic follow-up. Journal of the American College of Cardiology. 2004;43:1868–1872. doi: 10.1016/j.jacc.2003.12.050. [DOI] [PubMed] [Google Scholar]
- 114.Holmes DR, Reddy VY, Turi ZG, Doshi SK, Sievert H, Buchbinder M, Mullin CM, Sick P. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. 2009;374:534–542. doi: 10.1016/S0140-6736(09)61343-X. [DOI] [PubMed] [Google Scholar]
- 115.Friedman PA, Asirvatham SJ, Dalegrave C, Kinoshita M, Danielsen AJ, Johnson SB, Hodge DO, Munger TM, Packer DL, Bruce CJ. Percutaneous epicardial left atrial appendage closure: preliminary results of an electrogram guided approach. Journal of cardiovascular electrophysiology. 2009;20:908–915. doi: 10.1111/j.1540-8167.2009.01465.x. [DOI] [PubMed] [Google Scholar]
- 116.Singh SM, Dukkipati SR, d’Avila A, Doshi SK, Reddy VY. Percutaneous left atrial appendage closure with an epicardial suture ligation approach: a prospective randomized pre-clinical feasibility study. Heart Rhythm. 2010;7:370–376. doi: 10.1016/j.hrthm.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 117.Lee RJ, Bartus K, Yakubov SJ. Catheter-based left atrial appendage (LAA) ligation for the prevention of embolic events arising from the LAA: initial experience in a canine model. Circulation. Cardiovascular interventions. 2010;3:224–229. doi: 10.1161/CIRCINTERVENTIONS.109.914978. [DOI] [PubMed] [Google Scholar]
- 118.Bartus K, Bednarek J, Myc J, Kapelak B, Sadowski J, Lelakowski J, Yakubov SJ, Lee RJ. Feasibility of closed-chest ligation of the left atrial appendage in humans. Heart Rhythm. 2011;8:188–193. doi: 10.1016/j.hrthm.2010.10.040. [DOI] [PubMed] [Google Scholar]
- 119.Vaseghi M, Lux RL, Mahajan A, Shivkumar K. Sympathetic stimulation increases dispersion of repolarization in humans with myocardial infarction. Am J Physiol Heart Circ Physiol. 2012;302:H1838–1846. doi: 10.1152/ajpheart.01106.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tung R, Boyle NG, Shivkumar K. Catheter ablation of ventricular tachycardia. Circulation. 2011;123:2284–2288. doi: 10.1161/CIRCULATIONAHA.110.989079. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


