Abstract
With aging, most skeletal muscles undergo a progressive loss of mass and strength, a process termed sarcopenia. Aging-related defects in mitochondrial energetics have been proposed to be causally involved in sarcopenia. However, changes in muscle mitochondrial oxidative phosphorylation with aging remain a highly controversial issue, creating a pressing need for integrative approaches to determine whether mitochondrial bioenergetics are impaired in aged skeletal muscle. To address this issue, mitochondrial bioenergetics was first investigated in vivo in the gastrocnemius muscle of adult (6 months) and aged (21 months) male Wistar rats by combining a modular control analysis approach with 31P magnetic resonance spectroscopy measurements of energetic metabolites. Using this innovative approach, we revealed that the in vivo responsiveness (‘elasticity’) of mitochondrial oxidative phosphorylation to contraction-induced increase in ATP demand is significantly reduced in aged skeletal muscle, a reduction especially pronounced under low contractile activities. In line with this in vivo aging-related defect in mitochondrial energetics, we found that the mitochondrial affinity for ADP is significantly decreased in mitochondria isolated from aged skeletal muscle. Collectively, the results of this study demonstrate that mitochondrial bioenergetics are effectively altered in vivo in aged skeletal muscle and provide a novel cellular basis for this phenomenon.
Keywords: adenosine nucleotide translocator, affinity for ADP, energetics, in vivo, mitochondria, skeletal muscle aging
Introduction
Skeletal muscle aging is characterized by a progressive loss of muscle mass and strength, a process named sarcopenia (Rosenberg, 1997). In the elderly, sarcopenia progressively leads to a loss of autonomy and an increased risk of injury (Visser et al., 2005). As the elderly (over 60 years of age) population is rising steadily and is expected to reach 22% of the worldwide population in 2050 (UN, New York, World Population aging 2009), identifying the mechanisms behind sarcopenia is a major research priority. One of the leading hypothesis to explain sarcopenia is the mitochondrial theory of aging, which postulates that accumulation of mitochondrial dysfunctions with aging plays a causal role in muscle atrophy (Dirks et al., 2006). The generally accepted view of this theory is that, due to the reactive oxygen species (ROS) production inherent in respiratory chain activity, aging is accompanied by the accumulation of oxidative damages to mitochondrial biomolecules, including several components of the oxidative phosphorylation machinery and mitochondrial DNA (Dirks et al., 2006). This damage is thought to result in (i) exacerbation of mitochondrial ROS production; (ii) increased sensitivity of mitochondria to promote apoptosis; and (iii) impaired capacity of mitochondria to adequately match the cellular ATP demand (Dirks et al., 2006; Bratic & Trifunovic, 2010). All these interrelated processes are currently proposed to be responsible for aging-related loss of muscle mass and function.
Whereas several evidences support this theory, for example aging-related increase in ROS production (Capel et al., 2005) or activation of mitochondria-driven pathways of apoptosis in aging muscle (Marzetti et al., 2008), the implications of defective mitochondria energetics as muscle ages are widely debated (see (Hebert et al., 2010) for a detailed review). Indeed, skeletal muscle oxidative capacity, assessed in vivo by 31P magnetic resonance spectroscopy (MRS), was reportedly decreased with aging in different human muscles (Chilibeck et al., 1998; Conley et al., 2000), suggesting decreasing maximal mitochondrial oxidative phosphorylation activity with muscle aging. In contrast, using comparable methodology, other research groups did not find any modification of oxidative capacity in several muscles with aging (Kent-Braun & Ng, 2000; Wray et al., 2009).
Experiments conducted in vitro also provided conflicting results. Aging-related decrease in maximal oxidation rate was found in mitochondria isolated from skeletal muscle of mice (Mansouri et al., 2006), rats (Kumaran et al., 2005), and in permeabilized fibers obtained from biopsies of human skeletal muscle (Tonkonogi et al., 2003). Accordingly, the maximal capacity of ATP production was also found to decline with aging in mitochondria isolated from skeletal muscle of rats (Drew et al., 2003) and humans (Short et al., 2005). However, Rasmussen et al. (2003) did not find any modification of mitochondrial oxidative phosphorylation with aging in mitochondria isolated from human quadriceps muscle. In addition, the maximal ADP-stimulated oxidation rate was reportedly unchanged in isolated mitochondria (Chabi et al., 2008) and in permeabilized myofibers (Picard et al., 2010) prepared from aged rat muscle. Clearly, no consensus has yet been reached, regarding the evolution of mitochondrial function with skeletal muscle aging.
Several reasons can be put forward to explain this lack of consensus. First, the majority of data available in the literature regarding the effect of muscle aging on mitochondrial oxidative phosphorylation have been obtained in vitro or in situ, under experimental conditions very different from the in vivo environment of mitochondria. Concurrently, recent studies strongly indicate that in vitro isolation procedures may affect mitochondrial function per se, independently of aging (Picard et al., 2010). Second, in vivo studies have been almost exclusively conducted in humans, where several factors known to affect mitochondrial function (i.e., physical activity levels, nutritional status, pathologies, lifestyle, etc.) are highly heterogeneous and therefore difficult to control. Researchers must therefore face numerous confounding factors when working with the elderly population, which certainly contribute to the current lack of agreement in the field. Finally, all studies that have been conducted to elucidate the effects of aging on mitochondrial oxidative phosphorylation focused only on extreme conditions of mitochondrial activity [i.e., maximal respiration activity (state 3) and mitochondrial respiration in the absence of ATP synthesis (state 4) in vitro, and/or maximal rate of phosphocreatine resynthesis rate in vivo]. However, these extremes are rarely, if ever, encountered in vivo, and therefore, the implications of these results in the context of sarcopenia are extremely difficult to interpret.
In this regard, we recently showed that mitochondria isolated from the gastrocnemius (GAS) muscle of aged rats display an impaired response to ATP demand (altered control pattern) under low phosphorylation activities (Gouspillou et al., 2010). Because these low activities match ATP turnover most frequently encountered in vivo in daily living, this aging-related dysregulation of mitochondria might therefore have physiologic relevance.
Building from our previous results, the aim of this study was to determine whether this mitochondrial dysfunction effectively impairs skeletal muscle energetics in vivo in aged rats. To achieve this aim, we studied in vivo the energy metabolism of moderately contracting GAS muscle of young adult (6 months) and aged (21 months) Wistar rats. This was achieved using a modular control analysis (MoCA) approach combined with 31P MRS measurement of the energetic intermediates that we have recently developed (Diolez et al., 2007; Arsac et al., 2008). Using this innovative approach, we reveal for the first time in vivo that activation of mitochondrial oxidative phosphorylation in response to a contraction-induced increase in ATP demand is significantly reduced in aged skeletal muscle. In line with this in vivo defect of mitochondrial oxidative phosphorylation, we also show that mitochondria isolated from aged muscles are characterized by a significant reduction in their affinity for ADP.
Results
Effect of aging on rat morphometric parameters
As compared to young adults, aged rats were characterized by a significantly higher body weight (Table 1) and a markedly reduced GAS weight (approximately −20%). The sarcopenic index, defined as the GAS-to-body weight ratio, was significantly lower in the aged group, demonstrating that this muscle was affected by sarcopenia.
Table 1.
Effect of aging on rat morphometric parameters
| Adults (n = 7) | Aged (n = 5) | |
|---|---|---|
| Body weight (g) | 591.7 ± 62.1 | 656.6 ± 57.8 |
| Gastrocnemius weight (g) | 3.4 ± 0.3 | 2.8 ± 0.6* |
| Sarcopenic index (%) | 0.58 ± 0.02 | 0.43 ± 0.10** |
Results, obtained from adult (6 month) and aged (21 month) rats, are expressed as mean ± SD. Sarcopenic index corresponds to the gastrocnemius weight to the body weight ratio, expressed as percentage.
*P < 0.05, **P < 0.01 vs. adult group.
Effect of aging on muscle energetics in vivo
The major difficulty in studying a biologic system—such as muscle energetics—in an integrative way is to deal with the high level of complexity. To face this challenge, complex systems can be simplified by grouping related processes into large modules connected by a small number of explicit intermediates, and integrative approaches such as MoCA can be used to extract relevant information (Brand & Curtis, 2002). In the present study, we applied a method recently developed by our group, which allows the application of elasticity analysis [a subset of the MoCA approach (Diolez et al., 2007)] to study GAS muscle energetics (Arsac et al., 2008). For the present application, muscle energetics was defined as a two-module system—one module constituting the supply and the other the utilization of energy (energy demand). The energy supply (mainly composed of substrate and O2 delivery systems, glycolysis, and mitochondrial oxidative phosphorylation) and the energy demand (mainly composed during contraction of the actomyosin ATPases and ATP-dependent ion pumps of the sarcoplasmic reticulum and sarcolemmal membrane) are therefore connected by available cellular energy—phosphorylation potential (ΔGp)—whose variations may be assessed by monitoring changes in phosphocreatine and Pi concentrations ([PCr], [Pi]) under our conditions (Diolez et al., 2007; Arsac et al., 2008).
The principles of the MoCA approach (stemming from metabolic control analysis) state that the internal control of a complex biologic system like muscle energetics is the direct consequence of the ‘elasticity coefficients’, which are the sensitivity/reactivity of each module to the common intermediate (in this case ∆Gp) (Diolez et al., 2007). In other words, these elasticity coefficients quantify the activation/inhibition of module’s activity in response to any change in the intermediate. Therefore, the determination of the elasticity coefficients of supply and demand modules toward the intermediate ∆Gp (assessed by changes in [PCr]) may be determined from module’s response to adequate experimentally induced change in [PCR] (see Methods). As mitochondrial oxidative phosphorylation is a major component of the defined energy supply of contraction energetics, we quantified the elasticity of this module under submaximal levels of contraction. The experimental determination of this elasticity coefficient requires (i) the determination of the flux through the energy supply (determined by measurement of developed contraction during steady states); (ii) the quantification of the value of [PCr] (determined using 31P MRS); and (iii) the ability to induce reasonably selective modulations of the energy demand to trigger changes in the energetic intermediate (Diolez et al., 2007; Arsac et al., 2008).
Figure 1 shows typical experiments recorded on adult and aged rats which allowed the calculation of the energy-supply elasticity in the GAS muscle (Arsac et al., 2008). First, a MRS spectrum was recorded at rest to determine the basal level of [PCr] (and was subsequently used to normalize our data). The intensity of stimulation was then progressively increased to trigger an intensity of contraction corresponding to the reference steady state. The determination of the energy-supply elasticity was then performed by increasing the intensity of stimulation (Fig. 1). As only relative changes in the intermediate and the flux are required for the determination of elasticity coefficients (Brand, 1996), the energy-supply elasticity was calculated by dividing the relative change in contraction (flux) by the relative change in [PCr] between reference and increased intensity of stimulation steady states. Note in the examples provided in Fig. 1 that although the increase in force production caused by the increase in the stimulation intensity was slightly higher in the old animal, the corresponding drop in the PCr signal was much greater in the old rat as compared to its young adult counterpart.
Figure 1.
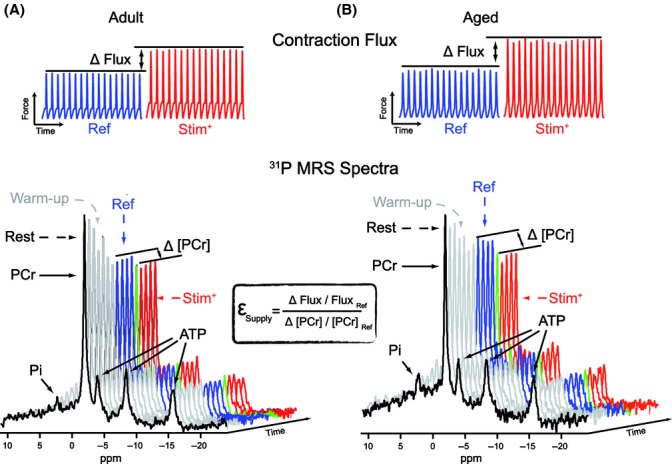
Typical phosphocreatine and contraction records carried out to calculate the elasticity of the energy-supply module in adult (A) and aged (B) animals. Muscle contraction and 31P MRS spectra were recorded simultaneously during each experiment. Magnetic resonance spectra were analyzed to assess steady values of energetic intermediates. First, an MRS spectrum was recorded at rest (black spectrum) to determine the basal level of energetic intermediates. The intensity of stimulation was then progressively increased (warm-up phase, gray spectra), to place the gastrocnemius muscle at an intensity of contraction corresponding to a reference steady state (Ref; spectra & contraction activity in blue). The determination of the energy-supply module elasticity was then performed by increasing the intensity of stimulation (Stim+; spectra & contraction activity in red). The green spectrum corresponds to a transition spectrum. The magnitude of the contraction signal was averaged over the corresponding period of time. Relative changes in contraction and [PCr] from the Ref to Stim+ steady states provided all the required data for the calculation of the energy-supply module elasticity. εSupply, elasticity of the energy-supply module; Pi, inorganic phosphate; ATP, adenosine triphosphate; PCr, phosphocreatine.
The results of these experiments are presented in Fig. 2A. For both adults and aged groups, the energy-supply elasticity (response) was decreasing when contraction intensity increased (leading to PCr depletion). As shown in Fig. 2A, whatever the metabolic activity, the elasticity of the energy-supply module is clearly reduced in aged muscles as compared to adults. Interestingly, this decrease in the energy-supply elasticity was more pronounced for very low contraction activity (0–10% of [PCr] depletion). As can be seen in Fig. 2B and C, this decrease in the elasticity of the energy supply in aged rats is mainly the result of a greater relative drop in [PCr] in response to the increase in stimulation intensity from the reference steady state, as the corresponding increase in force production was comparable between young and aged rats. Altogether, these results show that a greater drop in [PCr]—triggered by the activation of contraction—is required to elicit the same activation of energy-supply processes in aged rats. The similar [PCr]/[Pi] ratio between adult and aged rats, at rest and at the different reference steady states we studied (i.e., before the increase in stimulation intensity used to determine the energy-supply elasticity) (Fig. 2D), associated with the unchanged total creatine content previously reported in aged muscles by other investigators (Ermini & Verzar, 1968; Dudley & Fleck, 1984; Conley et al., 2000), clearly demonstrates that the decrease in energy-supply elasticity in aged rats arises from alteration(s) located within this module (rather than from changes in metabolite pools). Taken altogether, these results clearly highlight that the energy-supply module is markedly affected during muscle aging under low contraction intensities.
Figure 2.
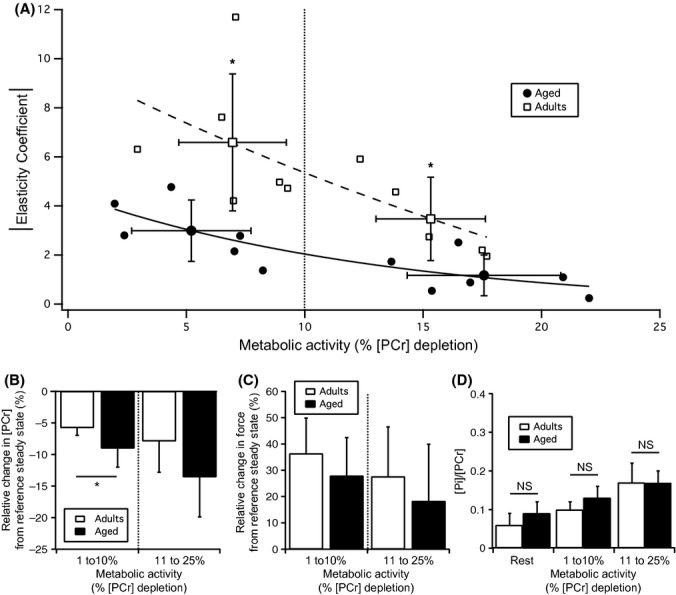
Effect of aging on the elasticity of the energy-supply module and on the PCr and Pi content in the gastrocnemius muscle. (A) The elasticity of the energy-supply module was determined under contraction intensities that elicited metabolic activities ranging from 2 to 25% depletion of [PCr] determined at rest. Each value presented in this figure corresponds to the elasticity of the energy-supply module determined in a single animal. Changes in elasticity as a function of metabolic activity were approximated by a mono-exponential function. (B) Relative change in [PCr] induced by the increase in stimulation intensity from reference steady state. (C) Relative change in force induced by the increase in stimulation intensity from reference steady state. (D) The [Pi]/[PCr] ratio determined at rest and under the different metabolic activity ranges corresponding to the reference steady states we studied was similar between adult and aged rats. Values are expressed as mean ± SD (n = 11 for adult and n = 12 for aged rats). *P < 0.05 vs. adult group.
It is highly unlikely that insufficient oxygen supply to GAS muscle in aged rats could explain the decrease in the elasticity of the energy-supply module. First, the cardiac output solicited during our experiments was undoubtedly very low. Second, changes in capillarization are also unlikely to contribute, especially considering the fact that muscle capillarization was reportedly unchanged or even slightly increased in skeletal muscle of aged rats (Hepple & Vogell, 2004). Further to this point, Musch et al. (2004) demonstrated that during submaximal exercise, the blood flow through the white and mixed region of the GAS muscle, the two regions mainly excited using our experimental setup (Arsac et al., 2008), is increased in aged rats as compared to young adults (Musch et al., 2004). Interestingly, it is well established that mitochondrial oxidative phosphorylation is the main source of energy supply under low and intermediate contractile intensities (Brooks, 1998). Based on this reasoning, our results strongly suggest that mitochondrial dysfunction plays a causal role in the decrease in the energy-supply elasticity with aging.
However, the possibility that changes in glycolytic activity may play a role cannot be ruled out at this stage. Activation of glycolysis is known to affect intramuscular pH during exercise. To this end, changes in intramuscular pH are usually assessed to investigate ATP production by glycolysis (Lanza et al., 2007). As a consequence, if alterations in glycolysis were causally involved in this decreased elasticity, the changes in intramuscular pH during the experimental determination of elasticity should have differed between adult and aged rats. As shown in Fig. 3, the intramuscular pH (assessed through the chemical shift of Pi on 31P spectra) (i) remained constant during the entire experiment; (ii) was identical between adult and aged rats; and (iii) always remained close to the intramuscular pH value at rest. These results indicate that the activation of glycolysis in our experiments was weak and similar between adult and aged rats. Therefore, our data show that the activation of mitochondrial oxidative phosphorylation in response to changes in [PCr] (i.e., changes in energy demand) is blunted in aged rats.
Figure 3.
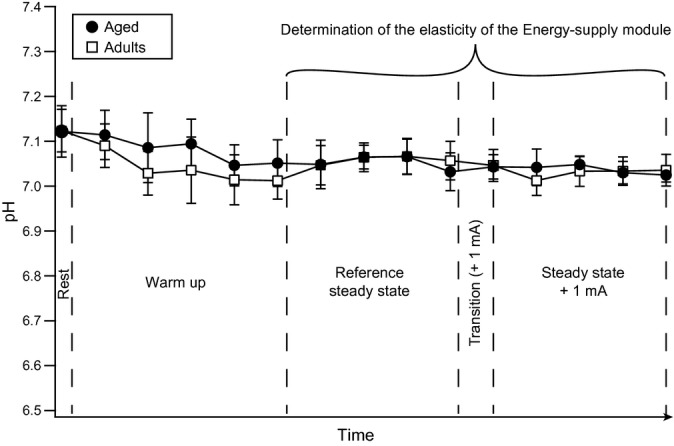
Changes in intramuscular pH during experimental determination of the energy-supply module elasticity. The chemical shift of Pi relative to PCr was used to assess the evolution of intramuscular pH during the experimental determination of the energy-supply elasticity. The different areas defined in this fig. correspond to the successive steps required for the experimental determination of the energy-supply module elasticity. Values are presented as mean ± SD (n = 11 for adult and n = 12 for aged rats).
In vitro study of the effect of aging on mitochondrial oxidative phosphorylation
Effect of muscle aging on mitochondrial content
To determine the effect of aging on mitochondrial content, citrate synthase (CS) activity was determined in the GAS muscle of young adult and aged rats. As shown in Table 2, CS activity was 25% lower in the GAS muscle of aged rats as compared to their young adult counterparts. This result therefore indicates a decrease in mitochondrial content with muscle aging.
Table 2.
Effect of muscle aging on CS activity
| Adults (n = 4) | Aged (n = 4) | |
|---|---|---|
| Citrate synthase activity (μmol min−1 g−1) | 18.0 ± 1.0 | 13.3 ± 3.1* |
Results, obtained from adult (6 month) and aged (21 month) rats, are expressed as mean ± SD.
P < 0.01 vs. adult group.
Effect of aging on oxidative phosphorylation affinity for ADP
Oxidative phosphorylation affinity for ADP is a parameter that reflects the capacity of mitochondria to respond to changes in ATP demand. As in vivo activation of mitochondria in response to an increase in the ATP demand was reduced in aged rats, we tested the effect of aging on mitochondrial affinity for ADP in mitochondria isolated from the GAS muscle. We previously emphasized that when dealing with energy balance the accurate calculation of mitochondrial affinity for ADP requires the determination of the phosphorylation rate (and not simply oxidation rate) dependency on ADP concentration under conditions where ADP is kept constant (Gouspillou et al., 2011). Isolated mitochondria, in which phosphorylation and oxidation rates can be easily determined simultaneously, were therefore a good choice for this study (Gouspillou et al., 2011).
According to our method, simultaneous measurements of oxidation and phosphorylation rates were carried out in mitochondria isolated from the GAS muscle of adult and aged rats for measured ADP concentrations ranging from 7 to 900 μm (Gouspillou et al., 2011). Results are shown in Fig. 4 and present very different kinetics for adult and aged rats. Maximal oxidation and phosphorylation rates (determined under the highest concentration of ADP) were significantly reduced in mitochondria isolated from aged rat muscle. Maximal oxidation and phosphorylation rates (determined under the highest concentration of ADP) were significantly reduced in mitochondria isolated from aged rat muscle (maximal oxidation rate: 392.8 ± 43.0 vs. 309.7 ± 42.0 nmolO2 × min−1 × mg−1 and maximal phosphorylation rate: 1596.1 ± 158.1 vs.1262.0 ± 140.4 nmolATP × min−1 × mg−1, for adult vs. aged rats, respectively; P < 0.01). From the kinetics shown in Fig. 4, the Km for ADP was determined for both oxidation (KmVox) and phosphorylation (KmVp) rates. In addition to their reduced maximal oxidative phosphorylation capacity mitochondria isolated from aged muscle were characterized by a significant increase in KmVox and KmVp (Fig. 4). These results demonstrate that the affinity of oxidative phosphorylation for ADP is reduced in the GAS of aged rats.
Figure 4.
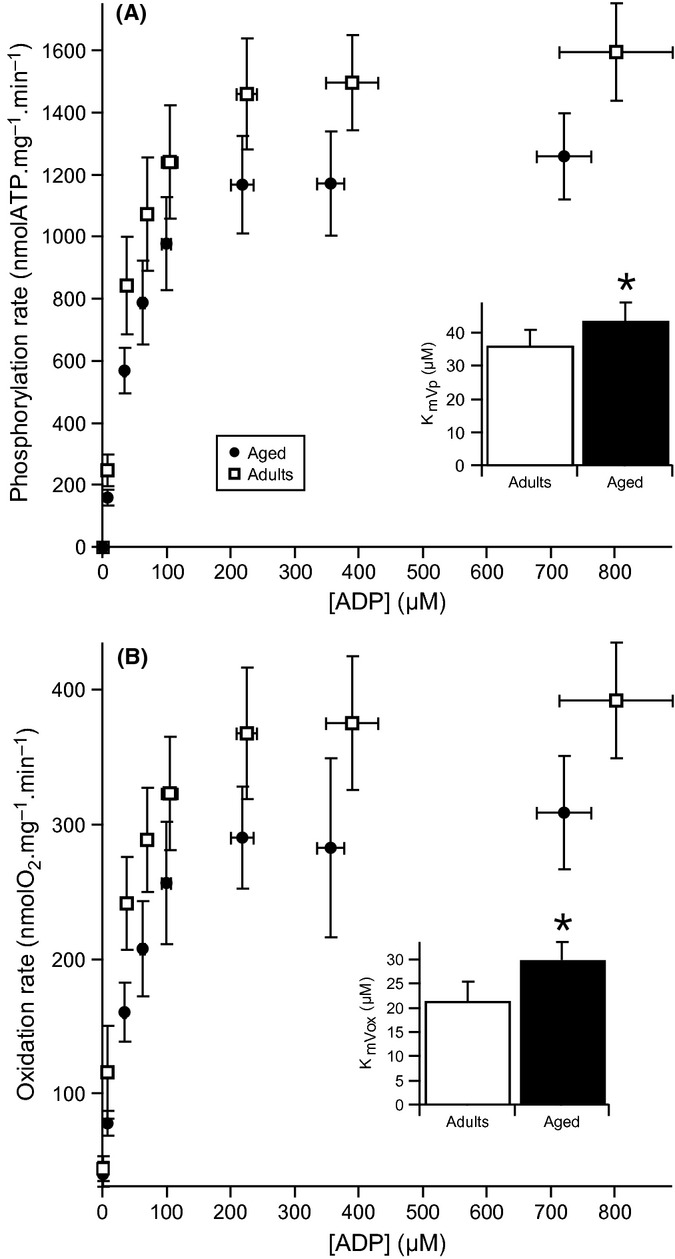
Dependence of oxidation and phosphorylation rates on ADP concentration in adult (A) and aged (B) gastrocnemius muscle mitochondria. Oxidation and phosphorylation rates were recorded simultaneously in mitochondria isolated from adult (n = 7) and aged (n = 5) muscles oxidizing glutamate + malate + succinate as substrates. Data were fitted using the Michaelis–Menten equation. Km for ADP was determined from both oxidation (KmVox) and phosphorylation (KmVp) rates. Data are presented as mean ± SD. *P < 0.05 vs. adult group.
Mitochondrial coupling efficiency
To define whether mitochondrial coupling efficiency was affected by skeletal muscle aging, we took advantage of the capabilities of our in vitro experimental setup allowing the simultaneous assessment of oxidation and phosphorylation rates. Mitochondrial coupling efficiency (ATP to O ratio) was therefore determined directly for each constant ADP concentration across the whole range of mitochondrial activity. As shown in Fig. 5A, neither maximal coupling efficiency nor dependence of coupling efficiency on the ADP concentration was affected in aged rats. Moreover, when the ADP to O ratio was plotted as a function of the phosphorylation rate, we found that aged mitochondria displayed a trend for a higher coupling efficiency for any given phosphorylation rate (Fig. 5B).
Figure 5.
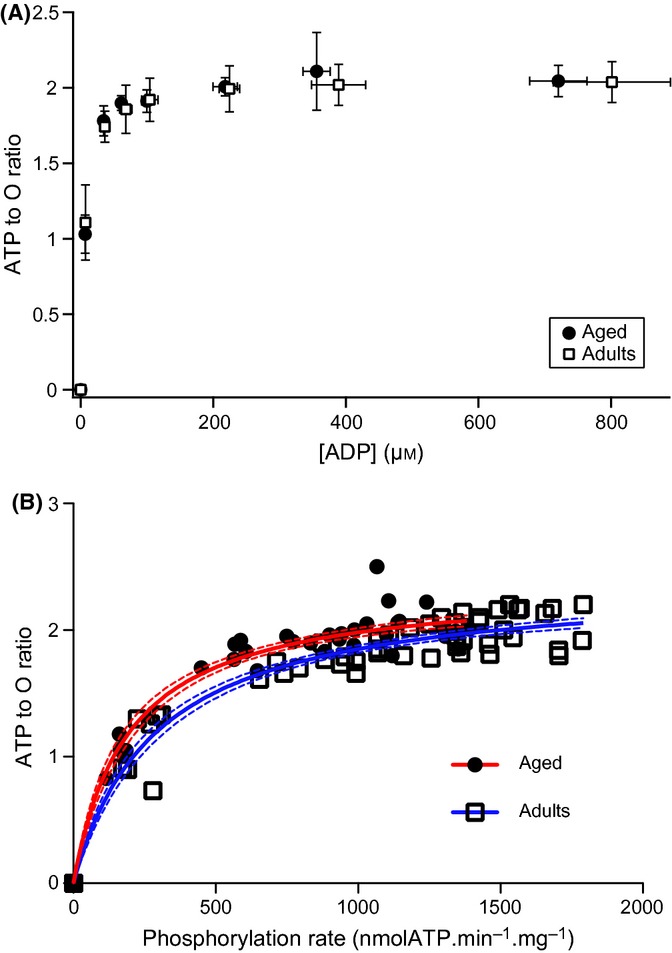
Changes in the ATP to O ratio as a function of ADP concentration and phosphorylation rate in adult and aged mitochondria. ATP to O ratio was determined by calculating the ratio of phosphorylation to oxidation rates. ATP to O ratios obtained from young and aged mitochondria were plotted as a function of (A) ADP concentration or (B) phosphorylation rates. Data for adult (n = 7) and aged (n = 5) rats are presented as mean ± SD in panel (A). Thick red (aged) and dashed blue (adults) curves in panel B were obtained by fitting data using the Michaelis–Menten equation. Thin dashes red (aged) and blue (adults) curved in panel B represent the 95% confidence interval of each curve fit.
Effect of aging on mitochondrial ANT content
Mitochondrial affinity for ADP primarily depends on the kinetic properties of phosphorylation and ATP channeling processes (ANT and ATP synthase). Interestingly, we have previously demonstrated that mitochondria isolated from the GAS muscle of aged rats exhibit an increased sensitivity to atractyloside, an ANT-specific inhibitor (Gouspillou et al., 2010). Therefore, we investigated whether changes in the ANT content could explain the decrease in mitochondrial affinity for ADP in aged muscle using Western blot analyses. To ensure accurate quantification of ANT content, results were normalized both per μg of protein loaded on the gels and per cytochrome c oxidase content, as the latter was reported unchanged in the aged GAS muscle (Lombardi et al., 2009). As shown in Fig. 6, no change in ANT content was found with muscle aging.
Figure 6.
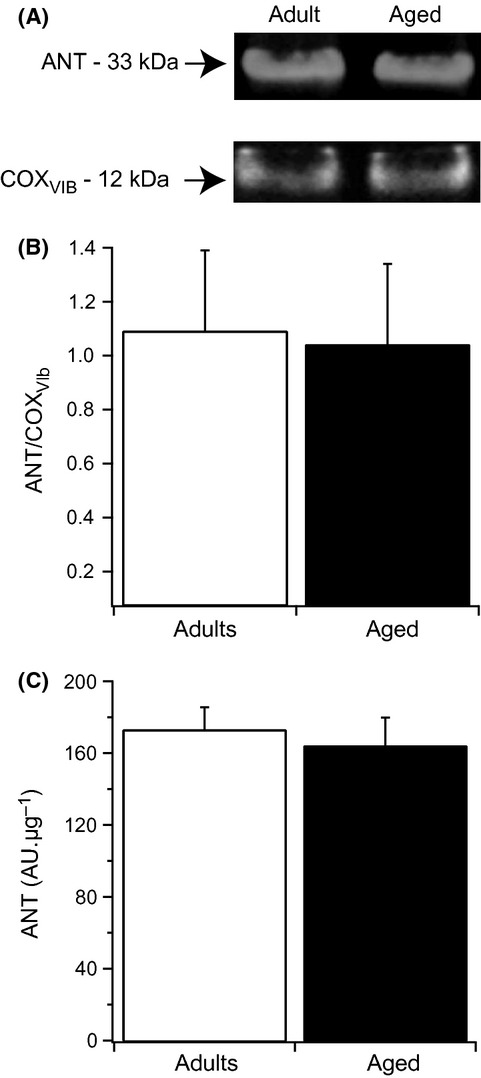
Effect of aging on mitochondrial ANT content. (A) Representative Western blot of ANT and cytochrome c oxidase obtained by the study of mitochondria isolated from adult and aged gastrocnemius muscle. (B) ANT content normalized cytochrome c oxidase content. (C) ANT content normalized to the mitochondrial protein quantity used for Western blot (10 μg). Data presented in (B) and (C) are expressed as mean ± SD (n = 4 per group).
Discussion
The aim of this study was to determine whether mitochondrial energetics is effectively impaired in aged skeletal muscle in vivo. To achieve this aim, we coupled a conceptual tool, MoCA, to the noninvasive measurement of energetic intermediates (Diolez et al., 2007; Arsac et al., 2008). Thanks to this innovative approach, we demonstrate that the elasticity of the energy-supply module is reduced in the GAS of aged rats under moderate levels of contraction. In other words, the decrease in the elasticity of the energy supply indicates that a greater drop in PCr level, concomitant with a greater rise in ADP, is required to induce equivalent activation of energy supply in aged muscle as compared to young. As we demonstrated here that glycolysis activation was negligible during our experiments, and because changes in O2 and substrate supply processes are unlikely to play a significant role under low contraction activities, our in vivo results strongly indicate that the activation of mitochondrial oxidative phosphorylation in response to an increase in ATP demand is markedly reduced with skeletal muscle aging.
Mitochondrial content is usually believed to decrease with muscle aging (Johnson et al., 2013), although this idea is debated (Picard et al., 2010). Based on the 25% decrease in CS activity we found in aged rats (Table 2), our results suggest that mitochondrial content was lower in our aged animals. Although this might be interpreted as a contributing factor to the decrease in the elasticity of the energy-supply module, we recently collected data indicating that variation in mitochondrial content do not necessarily relate to changes in the elasticity of the energy-supply module. Indeed, we found that although a 21-day exposure to hypoxia lead to a decrease in mitochondrial content (18% decrease in CS activity) in rat heart, it resulted in a fourfold increase in the elasticity of the energy-supply module (Calmettes et al., 2010). In addition, because elasticity coefficients are intrinsically linked to the module kinetics (Brand & Curtis, 2002), and because we only investigated very low and low contractile activities (inducing less than 25% depletion in [PCr], activities unlikely to require the maximal oxidative phosphorylation capacity), a decrease in mitochondrial content (or in maximal oxidative phosphorylation capacity) is unlikely to explain the decrease in the elasticity of the energy-supply module found in aged rats. Therefore, this decrease in the elasticity of the energy supply points toward the existence of an aging-related intrinsic change in mitochondrial function.
The in vitro study of mitochondrial bioenergetics performed in this study allowed us to identify the cellular basis of the in vivo impairment of mitochondrial bioenergetics in aged muscle. Indeed, the significant decrease in mitochondrial affinity for ADP observed in mitochondria isolated from aged GAS muscle is perfectly in line with the age-related reduction in the in vivo activation of mitochondrial oxidative phosphorylation in response to an increase in ATP demand (decrease in elasticity of energy supply). In fact, a decreased affinity for ADP indicates that a higher increase in ADP concentration is required to induce a given activation of mitochondrial oxidative phosphorylation. This reduced mitochondrial affinity for ADP in aged rats is also perfectly in line with the aging-related alteration in the regulation of oxidative phosphorylation that we previously reported (Gouspillou et al., 2010).
As opposed to previous studies that have either reported an aging-related decline (Tonkonogi et al., 2003; Kumaran et al., 2005; Marcinek et al., 2005) or increase in mitochondrial coupling efficiency (Kerner et al., 2001), no aging-related changes in this parameter were observed in the present study (Fig. 5). This result is in line with the unaltered proton leak kinetics we previously reported in similar aged rats (Gouspillou et al., 2010). Here, we even found a trend for a higher coupling efficiency for any given phosphorylation rate in mitochondria isolated from aged muscle (Fig. 5B). This result, indicating that a change in mitochondrial coupling efficiency is unlikely to explain our in vivo results, may be explained by the fact that aged mitochondria display a lower membrane potential for any given phosphorylation activity (Gouspillou et al., 2010) (a lower membrane potential implying a reduction in proton leak and therefore an increase in ATP to O ratio).
This modified mitochondrial response toward ATP demand we revealed here can have profound cellular consequences. For instance, defects in mitochondrial bioenergetics may explain the decrease in ATP content reported in mitochondria from the GAS muscle of aged rats (Drew et al., 2003) and the significant decline in ATP concentration with aging that was found in human muscle (Conley et al., 2000). Moreover, the decrease in mitochondrial affinity for ADP undoubtedly increases the ADP to ATP ratio and in turn may contribute to an increase in the AMP-to-ATP ratio through the activity of adenylate kinase (Hardie, 2004). In accordance with this, the AMP-to-ATP ratio was reportedly increased in the skeletal muscle of aged rats (Bastien & Sanchez, 1984). The AMP-to-ATP ratio is now well known to regulate the activity of AMP-activated protein kinase (Hardie, 2004). Interestingly, this protein kinase has already been implicated in the age-related loss of muscle mass of fast glycolytic muscles (Gordon et al., 2008), an effect mediated through a positive regulation of FOXO activity, which in turn promotes the expression of the atrophy-related ubiquitin ligases atrogin-1 and MuRF-1 (Gordon et al., 2008). Therefore, the age-related impairment of mitochondrial response to changes in ATP demand demonstrated here provides a bioenergetics basis to explain, at least in part, the age-related activation of some muscle atrophy pathways.
As discussed previously, the cellular basis for the in vivo alterations of mitochondrial bioenergetics in aged muscle involves a modification of mitochondrial affinity for ADP. Mitochondrial affinity for ADP is a bioenergetic parameter that greatly depends on ANT kinetic properties (Gouspillou et al., 2011). Interestingly, we recently showed that mitochondria isolated from aged GAS muscle exhibit a marked increase in atractyloside (a specific ANT inhibitor) sensitivity, therefore indicating that ANT function is modified during muscle aging (Gouspillou et al., 2010). Based on this finding, we have proposed that altered ANT function was involved in the aging-related changes in mitochondrial oxidative phosphorylation (Gouspillou et al., 2010). The decreased mitochondrial affinity for ADP revealed here therefore strengthens the potential role played by ANT in the aging-related alteration of mitochondrial bioenergetics. As the ANT content was unchanged in aged muscles (Fig. 6), a result in line with previous findings (Chabi et al., 2008; Marzetti et al., 2008), the aging-related change in atractyloside sensitivity (Gouspillou et al., 2010) therefore arises from an intrinsic change in ANT functional properties. Interestingly, oxidative modifications of ANT, quantified by the determination of ANT carbonyl content, were found to increase with aging in the flight muscle of the housefly (Yan & Sohal, 1998) and in rat skeletal muscle (Feng et al., 2008). Given the fact that an increase in ANT carbonyl content was previously shown to impair ANT activity (Yan & Sohal, 1998), this accumulation of ANT oxidative modifications with aging may therefore represent a molecular mechanism leading to the in vivo alteration of mitochondrial bioenergetics.
In summary, the results from this study demonstrate that mitochondrial bioenergetics are appreciably impaired in vivo in aged skeletal muscle, particularly under conditions of low activity, therefore in the range of daily living activities. Furthermore, these results indicate that the cellular mechanism underlying this in vivo impairment in mitochondrial energetics involves a decreased in mitochondrial affinity for ADP. Based on this decrease in affinity for ADP, associated with the increased atractyloside sensitivity we previously reported, we hypothesize that impairment in ANT function might be a central mechanism causing aging-related defects in mitochondrial bioenergetics. Further work is now required to clarify the role of changes in ANT function and to define the importance of mitochondrial bioenergetics defects in sarcopenia.
Experimental procedures
Animals
Experiments were conducted on male Wistar rats aged 6 and 21 months. Rats were housed in an environmentally controlled facility (12-h/12-h light/dark cycle, 22 °C) and received water and food ad libitum until in vitro experiments were performed. All experiments were conducted in agreement with the National and European Research Council Guide for the care and use of laboratory animals. P. Diolez has a permanent license to conduct experiments on animals by the Service Vétérinaire de la Santé et de la Protection Animale of the Ministère de l’agriculture et de la Forêt (03/17/1999, license number 3308010).
In vivo study of muscle energetics
Muscle stimulation
Plantar flexor muscles of rats in a supine position were stimulated (3 Hz; pulse duration: 220 μs) directly with transcutaneous surface electrodes located at the knee and heel levels (Arsac et al., 2008). These electrodes were connected to a Compex 2 stimulator (Compex Médical SA, Ecublens, Switzerland). Current intensity was set to 4 mA to study muscle energetics under low contraction activity. Using this electrostimulation setup, we previously demonstrated that the GAS muscle is virtually exclusively recruited (Arsac et al., 2008). Therefore, all the in vivo data presented in the present study were obtained in this specific muscle. MRS spectra acquisition was synchronized to electrical stimulation.
31P MRS
Rats were placed in a supine position into a supraconducting magnet (4.7T; 47/50 Biospec Avance magnetic resonance system, Bruker, Karlsruhe, Germany). The foot was positioned on the ergometer pedal, and the hindlimb was immobilized so that the lower limb was centered inside a 30-mm-diameter 1H Helmholtz imaging coil tuned to the 1H frequency (200.3 MHz).
A circular home-built transmitter–receiver probe (18 mm) was placed horizontally under the calf and was tuned to the 31P frequency (81.1 MHz). Field homogeneity was achieved locally in a 20 × 20 × 20 mm3 voxel using the water proton resonance, with typical line widths of 30–35 Hz.
31P free-induction decays (FIDs) (100 μs rectangular pulse, 60° flip angle at the center of the coil, 64 accumulations, 2.8 s recovery time, 3.3 kHz spectral width, 1024 data points) were acquired in 180 s blocks throughout the experimental protocol.
After Fourier transformation, the MRS spectra were routinely deconvolved into Lorentzian lines (Igor Pro; Wavemetrics, Lake Oswego, OR, USA) to determine the PCr and Pi peak areas. As only relative changes in [PCr] and the [Pi]/[PCr] ratio were determined, our analyses did not require an assumption on the [ATP]. Intracellular pH was determined using the chemical shift of Pi relative to PCr.
Contraction measurements
Contraction resulting from muscle electrical stimulation was measured with a home-built ergometer consisting of a foot pedal connected to a hydraulic piston. A hydraulic circuit filled with water connected the piston to a force transducer (MLT0699+ Powerlab; ADInstruments, Bella Vista, Australia), which was placed outside the magnet. The pedal was adjusted so that the foot was perpendicular to the leg. Changes in pressure induced by pedal stroke were recorded every 5 ms. The magnitude of strokes was computed as a function of time to provide the contraction signal. As only relative changes (in %) in contraction are required for elasticity analysis, contraction force was expressed as the voltage delivered by the sensor (μV).
Experimental determination of the energy-supply elasticity
Rats were anaesthetized by continuous inhalation of a gas mixture containing 1.5% isoflurane delivered via a face mask during a 70-min period (corresponding to the duration of the experiment in the magnet). Animals were then returned to the animal housing facility. As the study of mitochondrial function in vivo was the purpose of this study, the elasticity coefficient of energy supply was determined under low contraction activity. The typical design of each experiment is described in Fig. 1. Two 31P MRS spectra (spectra 1 and 2) were acquired before muscle stimulation began to assess [PCr] at rest. During spectra 3–7, muscle stimulation was progressively increased up to the desired current intensity to gently warm-up muscles. The system therefore reached the so-called reference steady state (state around which the elasticity of the energy-supply module was determined, spectra 8–11). A 1 mA increase in the electrical stimulation intensity was then applied to raise the energy consumption up. The steady state values of [PCr] and contraction forces developed during this stage when compared with the ‘reference’ were used for the calculation of the energy-supply elasticity. The elasticity coefficients in each individual animal were quantified by dividing the relative change in contraction by the relative change in [PCr] (Diolez et al., 2007).
In vitro study of mitochondrial bioenergetics
Determination of CS activity
Enzymatic activity levels of the mitochondrial enzyme CS were determined spectrophotometrically GAS homogenates as previously described (Picard et al., 2010). All samples were homogenized in a 200 mm Tris extraction buffer containing 50 mm triethanolamine and 1 mm EDTA at pH 7.4. Citrate synthase activity was measured by detecting the increase in absorbance at 412 nm in a 96-well plate at 30 °C, using a reaction buffer containing 2 μm acetyl-CoA, 200 μm 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB), 350 μm oxaloacetic acid, and 0.1% Triton-X100. Citrate synthase activity was calculated using a molar extinction coefficient of 13.6 L mol−1 × cm−1 for DTNB.
Isolation of skeletal muscle mitochondria
Male Wistar rats were anaesthetized by isoflurane inhalation and killed by intraperitoneal injection of pentobarbital (60 mg kg−1). The GAS muscle of the right leg was then dissected and washed in the isolation medium containing 100 mm sucrose, 180 mm KCl, 50 mm Tris, 5 mm MgCl2, 10 mm EDTA, and 0.1% (w/v) BSA (pH 7.2). Before homogenization, muscles were minced and exposed for 5 min to protease (2 mg mL−1 of isolation medium, Sigma P8038; Sigma-Aldrich, Saint-Quentin Fallavier, France). Mitochondria were extracted as previously described (Gouspillou et al., 2010). Mitochondrial protein concentration was determined by the Bradford method using BSA as standard.
Experimental determination of oxidation and phosphorylation rates and mitochondrial affinity for ADP
Mitochondrial bioenergetics were investigated as described in the study by Gouspillou et al. (2011). Briefly, oxygen consumption and ATP synthesis rates were monitored simultaneously during true steady states in a glass vessel (final volume 6 mL, 25 °C) in a medium containing 240 mm mannitol, 100 mm KCl, 1 mm EGTA, 20 mm MgCl2, 10 mm KH2PO4, and 0.1% (w/v) BSA (pH 7.2). Glutamate (5 mm) + malate (1 mm) + succinate (5 mm) were used as substrates in all experiments to reconstitute the tricarboxylic acid cycle function.
Oxidation rates were determined polarographically with a Clark electrode (Rank Brothers, Cambridge, UK). The use of the combined enzymatic system composed of glucose (5 mm), hexokinase (2.5 U mL−1, Sigma H4502), glucose-6-phosphatase dehydrogenase (2.5 U mL−1, Sigma G6378), NADP+ (1.6 mm) allowed the determination of phosphorylation rates as the NADPH production by this system is production is stoichiometrically linked to mitochondrial ATP synthesis rate. Changes in the rate of NADPH production were monitored at 340 nm by placing an optic fiber connected to a spectrophotometer in the oxygraphic vessel (Cary 50; Varian, Grenoble, France). Conversion of NADPH absorbance into NADPH concentration was performed using a molar extinction coefficient of 6.22 mm−1 cm−1. Bioluminescent assays were used to measure both ADP and ATP concentration during each experiment (Gouspillou et al., 2011). This method allowed the determination of the oxidation (KmVox) and phosphorylation (KmVp) affinities for ADP, as well as the determination of mitochondrial coupling efficiency.
Quantification of mitochondrial ANT content
Mitochondrial proteins (10 μg) were separated by SDS-PAGE (12.5% polyacrylamide) and transferred to PVDF membranes. ANT was detected with mouse monoclonal anti-ANT antibody (Mitosciences MSA02; 1:1000; Mitosciences, Eugene, OR, USA). Cytochrome c oxidase was detected with mouse monoclonal antibody raised against the Complex IV subunit VIb (Mitosciences MS413; 1:1000). The secondary antibodies were coupled to horseradish peroxidase. The immune complexes were detected using enhanced chemiluminescence.
Statistics
Experimental values are expressed as mean ± SD. Comparisons between adult and aged rats were made using unpaired bilateral Student’s t-tests. P values = 0.05 and 0.01 were considered significant.
Acknowledgments
We thank Dr. RT Hepple and Dr. M Picard (Dept of Kinesiology, McGill U.) for helpful discussion, and Dr. L Low (Dept of Dentistry, McGill U.) and K Dutkiewicz (Dept of Physiology, McGill U.) for careful reading of the manuscript. The authors have no conflict of interest to declare.
Author contributions
GG, IBM, LA, SM, JMF, ET, and PD involved in the conception and design; GG, RR, MB, MB, PD, DD, VT, VDA, and LA performed the collection and/or assembly of data, data analysis and interpretation; GG, IBM, LA, and PD wrote the manuscript. All authors approved the final version of the manuscript.
References
- Arsac LM, Beuste C, Miraux S, Deschodt-Arsac V, Thiaudiere E, Franconi JM, Diolez PH. In vivo modular control analysis of energy metabolism in contracting skeletal muscle. Biochem. J. 2008;414:391–397. doi: 10.1042/BJ20080280. [DOI] [PubMed] [Google Scholar]
- Bastien C, Sanchez J. Phosphagens and glycogen content in skeletal muscle after treadmill training in young and old rats. Eur. J. Appl. Physiol. Occup. Physiol. 1984;52:291–295. doi: 10.1007/BF01015212. [DOI] [PubMed] [Google Scholar]
- Brand MD. Top down metabolic control analysis. J. Theor. Biol. 1996;182:351–360. doi: 10.1006/jtbi.1996.0174. [DOI] [PubMed] [Google Scholar]
- Brand MD, Curtis RK. Simplifying metabolic complexity. Biochem. Soc. Trans. 2002;30:25–30. doi: 10.1042/0300-5127:0300025. [DOI] [PubMed] [Google Scholar]
- Bratic I, Trifunovic A. Mitochondrial energy metabolism and ageing. Biochim. Biophys. Acta. 2010;1797:961–967. doi: 10.1016/j.bbabio.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Brooks GA. Mammalian fuel utilization during sustained exercise. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1998;120:89–107. doi: 10.1016/s0305-0491(98)00025-x. [DOI] [PubMed] [Google Scholar]
- Calmettes G, Deschodt-Arsac V, Gouspillou G, Miraux S, Muller B, Franconi JM, Thiaudiere E, Diolez P. Improved energy supply regulation in chronic hypoxic mouse counteracts hypoxia-induced altered cardiac energetics. Plos One. 2010;5:e9306. doi: 10.1371/journal.pone.0009306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capel F, Rimbert V, Lioger D, Diot A, Rousset P, Mirand PP, Boirie Y, Morio B, Mosoni L. Due to reverse electron transfer, mitochondrial H2O2 release increases with age in human vastus lateralis muscle although oxidative capacity is preserved. Mech. Ageing Dev. 2005;126:505–511. doi: 10.1016/j.mad.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Chabi B, Ljubicic V, Menzies KJ, Huang JH, Saleem A, Hood DA. Mitochondrial function and apoptotic susceptibility in aging skeletal muscle. Aging Cell. 2008;7:2–12. doi: 10.1111/j.1474-9726.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- Chilibeck PD, McCreary CR, Marsh GD, Paterson DH, Noble EG, Taylor AW, Thompson RT. Evaluation of muscle oxidative potential by 31P-MRS during incremental exercise in old and young humans. Eur. J. Appl. Physiol. Occup. Physiol. 1998;78:460–465. doi: 10.1007/s004210050446. [DOI] [PubMed] [Google Scholar]
- Conley KE, Jubrias SA, Esselman PC. Oxidative capacity and ageing in human muscle. J. Physiol. 2000;526(Pt 1):203–210. doi: 10.1111/j.1469-7793.2000.t01-1-00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diolez P, Deschodt-Arsac V, Raffard G, Simon C, Dos Santos P, Thiaudiere E, Arsac L, Franconi JM. Modular regulation analysis of heart contraction: application to in situ demonstration of a direct mitochondrial activation by calcium in beating heart. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;293:R13–R19. doi: 10.1152/ajpregu.00189.2006. [DOI] [PubMed] [Google Scholar]
- Dirks AJ, Hofer T, Marzetti E, Pahor M, Leeuwenburgh C. Mitochondrial DNA mutations, energy metabolism and apoptosis in aging muscle. Ageing Res. Rev. 2006;5:179–195. doi: 10.1016/j.arr.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Drew B, Phaneuf S, Dirks A, Selman C, Gredilla R, Lezza A, Barja G, Leeuwenburgh C. Effects of aging and caloric restriction on mitochondrial energy production in gastrocnemius muscle and heart. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;284:R474–R480. doi: 10.1152/ajpregu.00455.2002. [DOI] [PubMed] [Google Scholar]
- Dudley GA, Fleck SJ. Metabolite changes in aged muscle during stimulation. J. Gerontol. 1984;39:183–186. doi: 10.1093/geronj/39.2.183. [DOI] [PubMed] [Google Scholar]
- Ermini M, Verzar F. Decreased restitution of creatine phosphate in white and red skeletal muscles during aging. Experientia. 1968;24:902–904. doi: 10.1007/BF02138640. [DOI] [PubMed] [Google Scholar]
- Feng J, Xie H, Meany DL, Thompson LV, Arriaga EA, Griffin TJ. Quantitative proteomic profiling of muscle type-dependent and age-dependent protein carbonylation in rat skeletal muscle mitochondria. J. Gerontol. A Biol. Sci. Med. Sci. 2008;63:1137–1152. doi: 10.1093/gerona/63.11.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon SE, Lake JA, Westerkamp CM, Thomson DM. Does AMP-activated protein kinase negatively mediate aged fast-twitch skeletal muscle mass? Exerc. Sport Sci. Rev. 2008;36:179–186. doi: 10.1097/JES.0b013e3181877e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouspillou G, Bourdel-Marchasson I, Rouland R, Calmettes G, Franconi JM, Deschodt-Arsac V, Diolez P. Alteration of mitochondrial oxidative phosphorylation in aged skeletal muscle involves modification of adenine nucleotide translocator. Biochim. Biophys. Acta. 2010;1797:143–151. doi: 10.1016/j.bbabio.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Gouspillou G, Rouland R, Calmettes G, Deschodt-Arsac V, Franconi JM, Bourdel-Marchasson I, Diolez P. Accurate determination of the oxidative phosphorylation affinity for ADP in isolated mitochondria. Plos One. 2011;6:e20709. doi: 10.1371/journal.pone.0020709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG. The AMP-activated protein kinase pathway–new players upstream and downstream. J. Cell Sci. 2004;117:5479–5487. doi: 10.1242/jcs.01540. [DOI] [PubMed] [Google Scholar]
- Hebert SL, Lanza IR, Nair KS. Mitochondrial DNA alterations and reduced mitochondrial function in aging. Mech. Ageing Dev. 2010;131:451–462. doi: 10.1016/j.mad.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepple RT, Vogell JE. Anatomic capillarization is maintained in relative excess of fiber oxidative capacity in some skeletal muscles of late middle-aged rats. J. Appl. Physiol. 2004;96:2257–2264. doi: 10.1152/japplphysiol.01309.2003. [DOI] [PubMed] [Google Scholar]
- Johnson ML, Robinson MM, Nair KS. Skeletal muscle aging and the mitochondrion. Trends Endocrinol. Metab. 2013;24:247–256. doi: 10.1016/j.tem.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent-Braun JA, Ng AV. Skeletal muscle oxidative capacity in young and older women and men. J. Appl. Physiol. 2000;89:1072–1078. doi: 10.1152/jappl.2000.89.3.1072. [DOI] [PubMed] [Google Scholar]
- Kerner J, Turkaly PJ, Minkler PE, Hoppel CL. Aging skeletal muscle mitochondria in the rat: decreased uncoupling protein-3 content. Am. J. Physiol. Endocrinol. Metab. 2001;281:E1054–E1062. doi: 10.1152/ajpendo.2001.281.5.E1054. [DOI] [PubMed] [Google Scholar]
- Kumaran S, Panneerselvam KS, Shila S, Sivarajan K, Panneerselvam C. Age-associated deficit of mitochondrial oxidative phosphorylation in skeletal muscle: role of carnitine and lipoic acid. Mol. Cell. Biochem. 2005;280:83–89. doi: 10.1007/s11010-005-8234-z. [DOI] [PubMed] [Google Scholar]
- Lanza IR, Larsen RG, Kent-Braun JA. Effects of old age on human skeletal muscle energetics during fatiguing contractions with and without blood flow. J. Physiol. 2007;583:1093–1105. doi: 10.1113/jphysiol.2007.138362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi A, Silvestri E, Cioffi F, Senese R, Lanni A, Goglia F, de Lange P, Moreno M. Defining the transcriptomic and proteomic profiles of rat ageing skeletal muscle by the use of a cDNA array, 2D- and Blue native-PAGE approach. J. Proteomics. 2009;72:708–721. doi: 10.1016/j.jprot.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Mansouri A, Muller FL, Liu Y, Ng R, Faulkner J, Hamilton M, Richardson A, Huang TT, Epstein CJ, Van Remmen H. Alterations in mitochondrial function, hydrogen peroxide release and oxidative damage in mouse hind-limb skeletal muscle during aging. Mech. Ageing Dev. 2006;127:298–306. doi: 10.1016/j.mad.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Marcinek DJ, Schenkman KA, Ciesielski WA, Lee D, Conley KE. Reduced mitochondrial coupling in vivo alters cellular energetics in aged mouse skeletal muscle. J. Physiol. 2005;569:467–473. doi: 10.1113/jphysiol.2005.097782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzetti E, Wohlgemuth SE, Lees HA, Chung HY, Giovannini S, Leeuwenburgh C. Age-related activation of mitochondrial caspase-independent apoptotic signaling in rat gastrocnemius muscle. Mech. Ageing Dev. 2008;129:542–549. doi: 10.1016/j.mad.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musch TI, Eklund KE, Hageman KS, Poole DC. Altered regional blood flow responses to submaximal exercise in older rats. J. Appl. Physiol. 2004;96:81–88. doi: 10.1152/japplphysiol.00729.2003. [DOI] [PubMed] [Google Scholar]
- Picard M, Ritchie D, Wright KJ, Romestaing C, Thomas MM, Rowan SL, Taivassalo T, Hepple RT. Mitochondrial functional impairment with aging is exaggerated in isolated mitochondria compared to permeabilized myofibers. Aging Cell. 2010;9:1032–1046. doi: 10.1111/j.1474-9726.2010.00628.x. [DOI] [PubMed] [Google Scholar]
- Rasmussen UF, Krustrup P, Kjaer M, Rasmussen HN. Experimental evidence against the mitochondrial theory of aging. A study of isolated human skeletal muscle mitochondria. Exp. Gerontol. 2003;38:877–886. doi: 10.1016/s0531-5565(03)00092-5. [DOI] [PubMed] [Google Scholar]
- Rosenberg IH. Sarcopenia: origins and clinical relevance. J. Nutr. 1997;127:990S–991S. doi: 10.1093/jn/127.5.990S. [DOI] [PubMed] [Google Scholar]
- Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, Nair KS. Decline in skeletal muscle mitochondrial function with aging in humans. Proc. Natl Acad. Sci. USA. 2005;102:5618–5623. doi: 10.1073/pnas.0501559102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonkonogi M, Fernstrom M, Walsh B, Ji LL, Rooyackers O, Hammarqvist F, Wernerman J, Sahlin K. Reduced oxidative power but unchanged antioxidative capacity in skeletal muscle from aged humans. Pflugers Arch. 2003;446:261–269. doi: 10.1007/s00424-003-1044-9. [DOI] [PubMed] [Google Scholar]
- Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, Simonsick EM, Harris TB. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J. Gerontol. A Biol. Sci. Med. Sci. 2005;60:324–333. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- Wray DW, Nishiyama SK, Monnet A, Wary C, Duteil S, Carlier PG, Richardson RS. Multiparametric NMR-based assessment of skeletal muscle perfusion and metabolism during exercise in elderly persons: preliminary findings. J. Gerontol. A Biol. Sci. Med. Sci. 2009;64:968–974. doi: 10.1093/gerona/glp044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan LJ, Sohal RS. Mitochondrial adenine nucleotide translocase is modified oxidatively during aging. Proc. Natl Acad. Sci. USA. 1998;95:12896–12901. doi: 10.1073/pnas.95.22.12896. [DOI] [PMC free article] [PubMed] [Google Scholar]


