Abstract
Nutrients including carbohydrates, proteins, lipids, vitamins, and minerals regulate various physiological processes and are essential for the survival of organisms. Reduced overall caloric intake delays aging in various organisms. However, the role of each nutritional component in the regulation of lifespan is not well established. In this review, we describe recent studies focused on the regulatory role of each type of nutrient in aging. Moreover, we will discuss how the amount or composition of each nutritional component may influence longevity or health in humans.
Keywords: aging, amino acid, carbohydrate, lipid, mineral, nutrient, protein, vitamin
Introduction
All organisms must obtain nutrients from their environments to live. These nutrients include organic chemicals, such as carbohydrates, proteins, lipids, and vitamins, and inorganic substances, such as minerals and water. The nutrients are essential for the maintenance of biological functions, including metabolism, growth, and repair. Interestingly, calorie restriction (CR), which is defined as a reduced intake of nutritional calories without malnutrition, has been shown to enhance the maintenance of biological systems and to increase lifespan (Kenyon, 2010). The molecular signaling pathways that mediate the effects of CR on longevity have been actively studied. In addition, many studies have specified which nutritional components contribute to aging, including early mammalian studies (Maeda et al., 1985; Masoro, 1985, 2002; Yu et al., 1985; Iwasaki et al., 1988a,b; Weindruch & Walford, 1988; Masoro et al., 1989), and this is currently an active area of investigation. Here, we will review recent findings regarding the effects of major dietary nutrients, namely carbohydrates, proteins and amino acids, lipids, and vitamins and minerals, on the lifespan of a diverse group of organisms, ranging from yeast to mammals. In addition to several excellent reviews on this topic (Piper et al., 2005, 2011; Tatar et al., 2014), our current review covers comprehensive ranges of nutritional components and their effects on aging in various organisms. Further, there is now increased understanding of the importance of diet for aging and age-related diseases in humans. Therefore, we will discuss the potential influences of these dietary components on human aging and age-related diseases.
Effects of carbohydrates on aging
Carbohydrates are organic compounds comprised of carbon, hydrogen, and oxygen. Carbohydrates act as signaling molecules, energy sources, and structural components. The importance of carbohydrates for human health is exemplified by the tight association between chronic metabolic diseases and carbohydrate-rich diets. Such diets have high glycemic indices, which result in rapid increases in blood glucose levels (Jenkins et al., 1981). In addition, recent studies indicate that several dietary carbohydrates directly influence lifespan in various organisms through diverse signaling pathways (Fig. 1).
Figure 1.
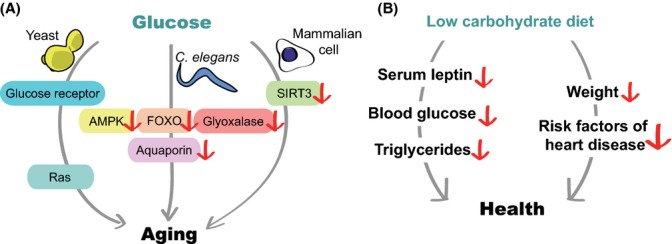
Glucose accelerates aging through various signaling pathways. (A) A high-glucose diet shortens lifespan in various organisms. In yeast, glucose decreases lifespan through the glucose receptor and Ras, components of a growth-promoting signaling pathway. In Caenorhabditis elegans, glucose downregulates pro-longevity proteins, such as AMP-activated protein kinase (AMPK), FOXO, and glyoxalase, resulting in short lifespan. Sirtuin 3 (SIRT3), an NAD-dependent protein deacetylase, mediates the effects of glucose on senescence in cultured mammalian cells. (B) Low-carbohydrate diets may improve human health by reducing several factors, including serum leptin, blood glucose, and triglycerides, which are associated with aging or metabolic defects. In addition, reduced carbohydrate intake decreases body weight and reduces the risk factors associated with heart disease.
Glucose alters lifespan through energy-sensing signaling pathways
Glucose, the primary energy source of most living organisms, is one of the best-studied carbohydrates that affect aging. Increased glucose intake accelerates aging in several model organisms, including yeast and Caenorhabditis elegans. Glucose-enriched diets shorten the lifespan of C. elegans by downregulating the activity of pro-longevity proteins, including AMP-activated protein kinase (AMPK), a FOXO transcription factor, and glyoxalase (Schulz et al., 2007; Lee et al., 2009; Schlotterer et al., 2009). Glucose consumption decreases the activity of AMPK, an energy sensor that regulates organismal lifespan. In contrast, treatment with a glucose analog, 2-deoxy-glucose, leads to glucose restriction, activation of AMPK, and longevity (Schulz et al., 2007). Glucose consumption decreases the activity of FOXO, a key downstream longevity transcription factor in the insulin/insulin-like growth factor-1 (IGF-1) signaling pathway (Lee et al., 2009). Reduced FOXO activity downregulates the aquaporin-1/glycerol channel and alters glycerol levels to shorten lifespan (Lee et al., 2009). Glucose-enriched diets also increase the level of methylglyoxal, a toxic advanced glycation end-product that is generated by nonenzymatic reactions during glucose metabolism, and this in turn reduces lifespan (Schlotterer et al., 2009). Moreover, recent studies show that the effect of glucose on the lifespan of C. elegans is modulated by a glucose transporter (Feng et al., 2013; Kitaoka et al., 2013) and pro-apoptotic genes (Choi, 2011). Thus, high dietary glucose appears to decrease the lifespan of C. elegans by influencing the activity of a variety of proteins that regulate lifespan and metabolism. The mechanisms through which glucose affects these factors coordinately or individually remain unclear.
Amounts of glucose negatively correlate with the lifespan of budding and fission yeasts (Roux et al., 2009; Weinberger et al., 2010). Glucose restriction, which is similar to dietary restriction (DR), increases the lifespan of the budding yeast Saccharomyces cerevisiae. However, excess glucose decreases lifespan through growth-promoting signaling proteins, such as Sch9, Tor1, and Ras (Weinberger et al., 2010). The glucose-sensing G-protein-coupled receptor Git3p (Welton & Hoffman, 2000) mediates the lifespan-shortening effect of glucose in the fission yeast Schizosaccharomyces pombe (Roux et al., 2009). Based on additional genetic data, glucose was proposed to activate Git3p, reducing lifespan through the activation of Gα and downstream Ras-cAMP/PKA signaling (Roux et al., 2009). Thus, the Ras pathway, one of the first signaling pathways to be implicated in the regulation of yeast lifespan (Chen et al., 1990), appears to play a central role in the effect of glucose on lifespan.
Glucose may also accelerate aging in mammals, although direct evidence is scarce. High concentrations of glucose in media accelerate the senescence of cultured human cells (Mortuza et al., 2013; Zhang et al., 2013). This pro-aging effect of glucose is associated with reduced expression of sirtuins, including SIRT3/sirtuin 3 (Mortuza et al., 2013; Zhang et al., 2013), a nicotinamide adenine dinucleotide (NAD)-dependent protein deacetylase (Haigis & Guarente, 2006). In addition, shRNA-mediated knockdown of SIRT3 accelerates senescence, whereas overexpression of SIRT3 suppresses glucose-induced cellular senescence (Zhang et al., 2013). Because glycolysis consumes NAD to produce NADH, the high-energy state caused by excess glucose may accelerate cellular senescence by downregulating the activity of sirtuins, such as SIRT3 (Kassi & Papavassiliou, 2008). It will be interesting to examine genetic mouse models of SIRT3 to determine whether this finding is consistent with organismal aging.
Carbohydrates that extend lifespan
In contrast to glucose, several other carbohydrates or carbohydrate metabolites, including trehalose, pyruvate, malate, fumarate, and N-acetylglucosamine (GlcNAc), have been shown to promote longevity in C. elegans (Honda et al., 2010; Mouchiroud et al., 2011; Edwards et al., 2013; Denzel et al., 2014). In particular, it is intriguing that a disaccharide trehalose is linked to longevity in yeast and C. elegans (Honda et al., 2010; Trevisol et al., 2011), because its monomer glucose decreases lifespan as described above. Trehalose feeding also increases stress resistance in C. elegans, which is consistent with the ability of trehalose to protect invertebrates from various stresses (Honda et al., 2010). Moreover, mutations that cause accumulation of trehalose promote fermentative capacity and extend the lifespan of yeast (Trevisol et al., 2011). Thus, trehalose appears to increase lifespan by acting as a general antistress sugar in invertebrates. In addition, GlcNAc, which is generated from glucose, increases the lifespan of C. elegans by improving the homeostasis of endoplasmic reticulum (ER) proteins (Denzel et al., 2014). Thus, trehalose and GlcNAc, which are metabolites of life-shortening glucose, appear to exert beneficial effects on lifespan in C. elegans.
Variable effects of carbohydrates on different model organisms
The effects of carbohydrates on aging are variable depending on species. In flies, the ratio of protein and carbohydrate (P:C) appears more important for lifespan regulation than individual nutrients (Mair et al., 2005; Min & Tatar, 2006; Lee et al., 2008; Skorupa et al., 2008; Fanson et al., 2009; Bruce et al., 2013). Likewise, low P:C diets are beneficial for health and aging in rodents (Solon-Biet et al., 2014). These studies point to crucial roles of proteins in lifespan regulation in flies and mammals (see the next section). Why are there differences among different species? First of all, we have to consider the possibility that different species have distinct physiological responses or diet-responsive signaling pathways to ingested nutrients depending on ecology. For example, responses to sugars may be more crucial for worms, but proteins are more important for flies in their natural habitats. Second, glucose is the most commonly used dietary carbohydrate for culturing C. elegans and yeast, whereas sucrose is used for Drosophila and rodents. In addition, studies using diets with defined nutrient composition are scarce in invertebrate models, because in most experimental paradigms, worms and flies, respectively, feed on Escherichia coli and yeast, which contain complex nutrients. Thus, future studies equipped with better understanding of the ecology of organisms and with defined diet systems will be crucial for addressing these questions.
Potential roles for dietary carbohydrates in human aging
Although the roles of dietary carbohydrates in human aging are unclear, clinical studies show that a low-carbohydrate diet is beneficial for human health (Rosedale et al., 2009). Administration of low-carbohydrate diets with high amounts of fats and adequate quantities of proteins significantly reduces body weight after 3 months (Rosedale et al., 2009). In addition, the human subjects show decreased levels of serum leptin, insulin, fasting glucose, and triglycerides, which are implicated in aging and metabolic defects. Low-carbohydrate diets also reduce body weight and several risk factors for heart disease (Foster et al., 2003). Conversely, high glycemic load diets enriched with carbohydrates positively correlate with age-related diseases including diabetes and heart diseases (Aston, 2006; Barclay et al., 2008). Thus, a low-carbohydrate diet may delay aging in humans by preventing metabolic diseases and improving general health.
Effects of dietary proteins and amino acids on aging
Proteins and amino acids are major biological macromolecules that serve as structural constituents, catalysts for enzymatic reactions, and energy sources. Many studies show that dietary proteins generally act as lifespan-limiting factors (Fig. 2). Deprivation of certain essential amino acids extends the lifespan of several model organisms, including Drosophila and mice (Fig. 2).
Figure 2.
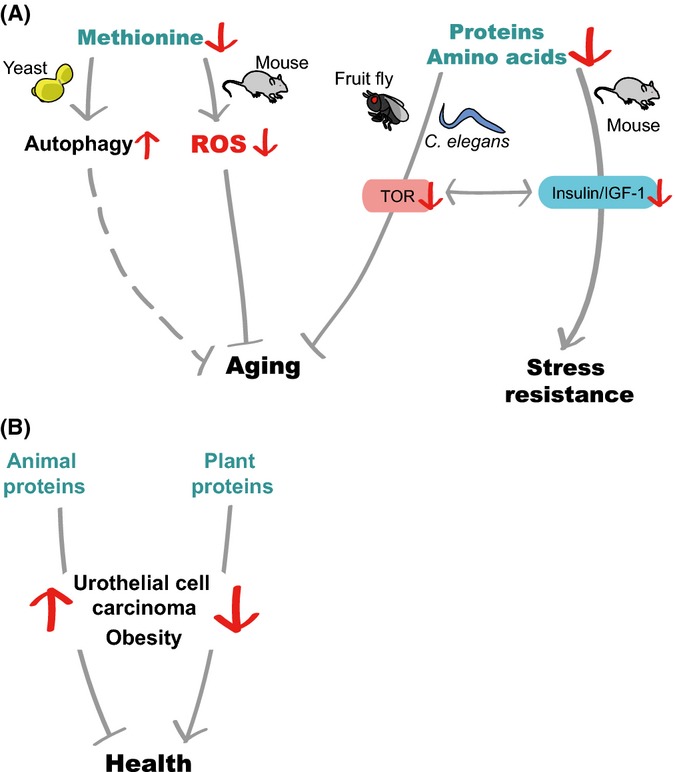
Restriction of amino acids or proteins increases stress resistance and influences longevity in various model organisms. (A) In yeast, methionine restriction decreases translational capacity and increases autophagy, indirectly interfering with aging. In addition, methionine restriction increases the lifespan of mice, possibly by downregulating the levels of reactive oxygen species (ROS). Dietary restrictions of protein intake increase the lifespan of various insects, including Drosophila. In Caenorhabditis elegans, reduced protein intake extends lifespan by inhibiting the insulin/insulin-like growth factor-1 (IGF-1) and target of rapamycin (TOR) signaling pathways. In mice, restrictions on the intake of protein or specific amino acids decrease oxidative stress by reducing insulin/IGF-1 signaling. (B) The levels of animal proteins positively correlate with the risk of urothelial cell carcinoma and obesity, whereas the levels of plant proteins exhibit a negative correlation.
The contribution of dietary proteins to animal aging
Studies in various model animals indicate a general negative correlation between the amounts of dietary proteins and lifespan. It is difficult to separate dietary proteins and carbohydrates from an essential diet. Therefore, many studies compared the effects of these two types of nutrients on aging by changing the dietary P:C ratio. Studies using Queensland fruit flies (Fanson et al., 2009), Mexican fruit flies (Carey et al., 2008), Drosophila melanogaster (Min & Tatar, 2006; Lee et al., 2008; Bruce et al., 2013), and field crickets (Maklakov et al., 2008) show that a low-protein/high-carbohydrate diet is associated with long lifespan; however, the overall caloric intake had minimal effects on lifespan (Mair et al., 2005). Similarly, low-protein/high-carbohydrate diets are linked to health and longevity in mice (Solon-Biet et al., 2014). In Drosophila, insulin-like peptides (dilps) have been shown to mediate the effects of the P:C ratio on lifespan by regulating target of brain insulin (tobi), which encodes an α-glucosidase (Buch et al., 2008). Reduced protein intake also appears to extend lifespan by inhibiting the insulin/IGF-1 or target of rapamycin (TOR) signaling pathways, which may reduce the levels of proteins with oxidative damage (Kapahi et al., 2004; Meissner et al., 2004; Sanz et al., 2004; Buch et al., 2008).
Despite a lack of direct evidence linking protein intake to human longevity, the source of dietary protein may affect human health. A large-scale, long-term study performed on European subjects indicates that high animal-protein intake positively correlates with the risk of developing urothelial cell carcinoma, whereas high plant-protein intake negatively correlates with the risk (Allen et al., 2013). This study also suggests that IGF-1 is a risk factor for the development of urothelial cell carcinoma in the setting of high animal-protein intake. A study of senior population reveals that subjects aged 50–65 that consumed high amount of protein had a 75% increase in overall mortality and fourfold increase in cancer-related death risk (Levine et al., 2014). This harmful effect seems attenuated by plant-derived protein diet. Another study that examined excess weight and obesity in Belgium indicates that the consumption of animal protein increases weight gain, whereas intake of plant protein is negatively associated with excess weight and obesity (Lin et al., 2011). Further, a nutritional investigation study demonstrates that a soy-based, low-calorie diet significantly reduces total serum cholesterol and body fat percentage in obese people compared with those achieved with a traditional, low-calorie diet (Liao et al., 2007). Although the mechanisms remain elusive, these studies reveal potential health benefits from diets that are enriched for plant proteins. Interestingly, plant proteins contain considerably lower methionines than animal proteins (McCarty et al., 2009), and this low methionine content may underlie the beneficial effects of dietary plant proteins (see next paragraph).
Roles of specific amino acids in longevity
In addition to the effects of overall proteins, many studies have determined the effects of specific dietary amino acids on lifespan. Under low amino acid status, methionine restriction increases lifespan in Drosophila by downregulating TOR signaling (Lee et al., 2014). Restriction of methionine extends lifespan in a variety of rat strains with different pathological backgrounds, suggesting that methionine deficiency alters the rate of aging rather than fixing a specific pathological defect (Zimmerman et al., 2003). Methionine restriction significantly extends the mean and maximum lifespan of mice, even when the experiments are conducted in 12-month-old animals (Miller et al., 2005; Sun et al., 2009). Methionine-restricted mice display physiological changes, such as reduced levels of insulin, IGF-1, and glucose, similar to those observed in calorie-restricted mice. However, gene expression profiles of methionine-restricted and calorie-restricted mice do not significantly overlap. Thus, these two dietary regimens may affect longevity through partly independent pathways. Methionine restriction lengthens the lifespan of male Wistar rats and decreases the production of mitochondrial reactive oxygen species (ROS) and DNA damage (Sanz et al., 2006; Caro et al., 2009; Sanchez-Roman et al., 2012). Lifespan extension in C. elegans due to treatment with metformin, a well-known antidiabetes drug, and mutations in metr-1/methionine synthase is associated with decreased levels of internal methionine (Cabreiro et al., 2013). However, several studies suggest that methionine has a positive impact on longevity. Methionine-supplemented casein- or soy-protein diets significantly lengthen the lifespan of spontaneously hypertensive rats that are prone to developing strokes (Gilani et al., 2006). In addition, methionine supplementation does not shorten long lifespan in Drosophila with DRs but does restore fecundity (Grandison et al., 2009). In contrast, supplementation with all kinds of amino acids or essential amino acids suppresses DR-induced longevity. Methionine restriction also causes a slight decrease in the average lifespan but does not affect reproductive fitness in Drosophila (Zajitschek et al., 2013). Thus, other amino acids, in addition to methionine, appear to have roles in lifespan regulation. Consistent with this concept, tryptophan restriction increases the lifespan of mice (De Marte & Enesco, 1986) and Evans rats (Segall & Timiras, 1976). Further, tryptophan restriction promotes resistance to surgical stress in mouse models of ischemia–reperfusion injury (Peng et al., 2012).
Dietary amino acid composition affects lifespan by regulating various nutrient-sensing signaling pathways. In yeast, eIF2α kinase and GCN2, which directly bind to uncharged cognate transfer RNAs (Wek et al., 1995; Dong et al., 2000), and TOR pathway components mediate longevity by acting as cellular amino acid sensors (Gallinetti et al., 2013). TOR signaling is inhibited and GCN2 is activated by reduced levels of internal amino acids; this inhibits overall protein translation and increases the translation of specific proteins involved in longevity (Gallinetti et al., 2013). In addition, restriction of dietary tryptophan protects mice from renal and hepatic ischemic injury and reduces inflammation in a Gcn2-dependent manner in association with reduced serum IGF-1 (Peng et al., 2012). Longevity of yeast due to methionine restriction appears to be mediated by TOR signaling (Laxman et al., 2013) and autophagy (Sutter et al., 2013), a process that recycles cellular components during nutrient deprivation. The anti-aging effects of CR are largely conserved from nematodes to primates. Therefore, it is worth investigating whether the mechanisms through which amino acid restriction promotes healthy and long lifespan are also evolutionarily conserved.
Dietary lipids exert various effects on aging
Dietary lipid components, including fatty acids, phospholipids, cholesterol, and glycerides, constitute the main structures in biological membranes. In addition, dietary lipids influence organismal physiology, including aging. A high-fat diet (HFD) is generally associated with increased mortality and increased incidence of many metabolic diseases, including type II diabetes and cardiovascular problems (Schrager et al., 2007; Honda et al., 2007) (Fig. 3). However, some specific lipids are beneficial for health and possibly longevity.
Figure 3.
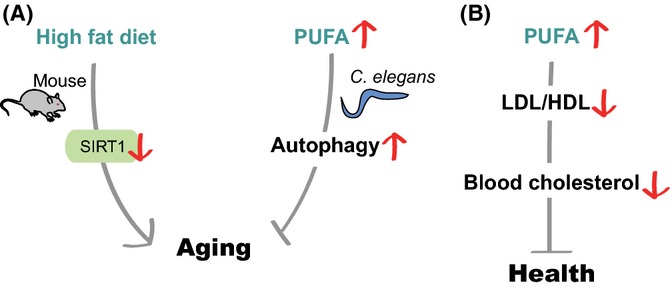
The amount and composition of dietary lipids influence organismal longevity. (A) In mice, a high-fat diet (HFD) inactivates SIRT1 and shortens lifespan. The composition of dietary fatty acids is critical for animal health. For example, ω-6 poly-unsaturated fatty acids (PUFAs) activate autophagy to promote longevity in Caenorhabditis elegans and probably in mammals. (B) PUFA-enriched diets decrease the ratio of low-density lipoprotein (LDL) to high-density lipoprotein (HDL); this results in reduced levels of blood cholesterol and improves health by ameliorating aging-associated diseases.
Metabolic regulators including SIRT1 counteract the effects of HFD on metabolic dysfunction and lifespan
Genetic factors that regulate HFD-induced pathology include SIRT1 (sirtuin 1, an NAD-dependent protein deacetylase), AMPK, peroxisome proliferator-activated receptors (PPARs), sterol regulatory element-binding protein 1 (SREBP-1), carbohydrate-responsive element-binding protein (ChREBP), superoxide dismutase 3 (SOD3), cysteine-aspartate protease-1 (caspase-1), and others (Lomb et al., 2010; Zadra et al., 2010; Jeon & Osborne, 2012; Dixon et al., 2013; Filhoulaud et al., 2013; Cui et al., 2014; Grygiel-Gorniak, 2014). Among them, SIRT1 is one of the best-studied factors mediating the effects of HFD on metabolism and lifespan in mammals. SIRT1 acts as a key cellular sensor for nutrient availability and regulates the activities of substrate proteins (Haigis & Guarente, 2006). Upregulation of SIRT1 improves glucose tolerance and insulin sensitivity in response to a HFD (Banks et al., 2008; Pfluger et al., 2008). Conversely, white-adipose-tissue-specific SIRT1-knockout mice display metabolic dysfunctions, such as insulin resistance, increased body weight, and excess levels of fat in high-fat-feeding conditions (Chalkiadaki & Guarente, 2012). Treatment with SIRT1-activating small molecules, including resveratrol and SRT1720, prevents adverse effects of HFD on metabolism and lifespan (Baur et al., 2006; Lagouge et al., 2006; Feige et al., 2008; Minor et al., 2011; Price et al., 2012). Despite the controversy regarding resveratrol as a direct SIRT1 agonist (Pacholec et al., 2010), the beneficial effects of resveratrol and SRT1720 largely disappear in SIRT1-knockout mice (Minor et al., 2011; Price et al., 2012). Further, resveratrol or SRT1720 treatment improves mitochondrial biogenesis and function via peroxisome proliferator-activated receptor gamma coactivator-1 alpha (PGC-1α) and estrogen-related receptor alpha (ERRα) (Baur et al., 2006; Lagouge et al., 2006; Feige et al., 2008; Price et al., 2012). Thus, enhanced SIRT1 activity may improve organismal survival in the context of HFD by upregulating genes that enhance mitochondrial function and reducing excess energy storage.
Dietary lipid composition and organismal lifespan
The composition of dietary lipid has dramatic effects on the level of blood cholesterol, which is crucial for the health of mammals. Diets enriched in unsaturated fatty acids lead to reduced blood levels of harmful low-density lipoproteins and increased levels of protective high-density lipoproteins (Mensink et al., 2003). Consistently, diets enriched in natural unsaturated fatty acids lower blood pressure, improve insulin sensitivity, and reduce the risks of cardiovascular and metabolic diseases (Summers et al., 2002; Appel et al., 2005). In contrast, dietary trans-fats (unsaturated fatty acids with trans-isomers) trigger inflammatory responses, which increase the risks of developing cardiovascular and metabolic diseases (Mozaffarian et al., 2004; Mozaffarian, 2006a,b; Riserus et al., 2009). Dietary saturated fatty acids are thought to be harmful to animal health, but this remains controversial (Siri-Tarino et al., 2010). Somewhat surprisingly, dietary cholesterol has been shown to marginally impact blood cholesterol levels (Fernandez, 2006). Overall, the composition of dietary lipid appears to be critical for blood cholesterol levels and may subsequently affect metabolic diseases and organismal lifespan.
Several studies indicate that polyunsaturated fatty acids (PUFAs) prevent aging-associated diseases and promote longevity. For example, arachidonic acids, which are omega (ω)-6 PUFAs, induce apoptosis of cancer cells (Cao et al., 2000). In addition, dietary arachidonic acids and eicosapentaenoic acids, which are ω-3 PUFAs, alleviate age-dependent neurodegeneration by increasing the expression of genes that are crucial for neurogenesis, neurotransmission, and neural connectivity (Das, 2008). In C. elegans, ω-6 PUFA feeding increases lifespan and resistance against nutrient deprivation by inducing autophagy (O'Rourke et al., 2013). In addition, ω-6 PUFAs activate autophagy in cultured mammalian cells, raising the possibility that similar life-extending mechanisms exist in mammals (O'Rourke et al., 2013).
Dietary lipids may affect mammalian health and longevity by altering the compositions of body fat and cellular membranes (Pamplona et al., 1998; Mitchell et al., 2007; Hulbert et al., 2008; Hulbert, 2010). Membrane PUFA levels are relatively low in the long-lived naked mole rat (Heterocephalus glaber) and the short-beaked echidna (Tachyglossus aculeatus) (Mitchell et al., 2007; Hulbert, 2010). This raises the possibility that membrane PUFA levels are linked to longevity. Consistent with that possibility, offspring from humans with long lifespan have low levels of PUFAs in the membranes of erythrocytes (Puca et al., 2008). Saturated fatty acids and monounsaturated fatty acids are generally more resistant to oxidative damage than that of PUFAs with multiple double bonds (Halliwell & Gutteridge, 1999; Hulbert et al., 2008). Thus, opposite from their potential role as dietary lipids, low levels of PUFAs in the membranes may be beneficial for longevity and health.
Effects of vitamins and minerals on aging
Although vitamins and minerals are not generally considered energy sources, these essential nutrients act as cofactors for diverse biological processes, such as mitochondrial energy metabolism and hormonal signaling (Ames et al., 2005). Humans cannot synthesize minerals or most vitamins; therefore, these must be supplied through dietary consumption. Deficiencies of essential vitamins and minerals can impair biological functions and promote the development of various diseases. Many studies indicate that vitamins and minerals also influence organismal lifespan (Fig. 4).
Figure 4.
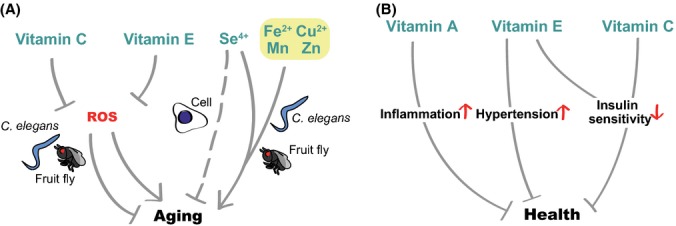
Effects of dietary vitamins and minerals on aging. (A) Vitamin C and vitamin E affect aging by acting as anti-reactive oxygen species (ROS) agents, which in turn decreases or increases lifespan in a context-dependent manner. In the case of minerals, supplementation with selenium (Se4+) delays cellular senescence, whereas dietary Se4+, Fe2+, Cu2+, Mn, and Zn confer short lifespan in Caenorhabditis elegans and/or fruit flies. (B) Implications for vitamins and minerals in human aging. Vitamin A increases the level of inflammation in patients with multiple sclerosis. Vitamin E increases blood pressure in patients with type II diabetes. Supplementation with vitamin E and C reduces insulin sensitivity by decreasing oxidative stress.
Many vitamins and minerals influence aging by acting as antioxidants
Studies have shown that dietary vitamins increase lifespan in various organisms primarily by functioning as antioxidants. For example, vitamin E/tocopherol intake significantly increases the lifespan of rotifers, nematodes, and fruit flies (Miquel et al., 1982; Sawada & Enesco, 1984; Harrington & Harley, 1988; Navarro et al., 2005). Vitamin E also increases the replicative lifespan of cultured adrenocortical cells and protects these cells from DNA-strand breaks in peroxide-treated conditions (Hornsby & Harris, 1987). Vitamin P/hesperidin increases the lifespan of yeast by reducing ROS (Sun et al., 2012). Supplementation of vitamin C/ascorbic acid, a well-known antioxidant, increases the lifespan of the bean beetle Callosobruchus maculatus (Garg & Mahajan, 1993). Although vitamin C feeding does not change the lifespan of D. melanogaster, vitamin C content declines with age in flies, suggesting that decreased vitamin C may be an indicator of aging (Massie et al., 1991). Furthermore, diets that include vitamin C rescue the short lifespan of wrn-1 (Werner helicase 1) mutant C. elegans by reducing the high levels of ROS and increasing the low levels of ATP in these mutant animals (Dallaire et al., 2012). Many members of the vitamin B family also lengthen the lifespan of flies, Zucker fatty rats, and C. elegans (Massie et al., 1993; Preuss et al., 2011; Schmeisser et al., 2013). For example, supplementation with vitamin B3 (nicotinic acid and nicotinamide) lengthens the lifespan of C. elegans through SIR-2.1, a worm homolog of SIRT1 (Schmeisser et al., 2013). These studies confirm the public belief that vitamins are generally beneficial for health, mostly because they moderate levels of ROS.
Although vitamins are generally considered to have beneficial effects on health, there is increasing evidence that vitamins also reduce lifespan. The antioxidant functions of vitamin C/ascorbic acid decrease the long lifespan conferred by mildly increased ROS in C. elegans (Schulz et al., 2007; Gomez-Cabrera et al., 2008; Yang & Hekimi, 2010). Feeding vitamin C and/or E shortens lifespan in the phlebotomine sand flies Lutzomyia longipalpis (Diaz-Albiter et al., 2011) and in wild-derived voles (Selman et al., 2013). In addition, vitamin C feeding reduces the enhanced mitochondrial functions caused by exercise in rats (Gomez-Cabrera et al., 2008). This is associated with reduced expression of PGC-1, nuclear respiratory factor 1 (NRF-1), and mitochondrial transcription factor A (mTFA), which are key transcription factors required for mitochondrial biogenesis. Consistently, vitamin C and E supplementation decreases oxidative stress but inhibits the beneficial effects of physical exercise on enhanced insulin sensitivity in humans (Ristow et al., 2009). Vitamin E intake causes hypertension in patients with type 2 diabetes (Ward et al., 2007). Moreover, a mega-dose of vitamins and minerals mildly increases human mortality (Lesperance et al., 2002). A meta-analysis of 385 publications indicates that overall levels of antioxidant supplementation positively correlate with mortality (Bjelakovic et al., 2007). In the case of multiple sclerosis, supplementation with vitamin A for 6 months increases the level of C-reactive protein (CRP), which is indicative of the level of inflammation (Jafarirad et al., 2013). Because moderate levels of ROS are beneficial for health and longevity (Heidler et al., 2010; Lee et al., 2010; Yang & Hekimi, 2010), antioxidant vitamins may interfere with the beneficial roles of ROS. In addition to these antioxidant vitamins, vitamin B9/folate displays a negative correlation with longevity in certain conditions, as reduced dietary vitamin B9 extends lifespan in C. elegans (Virk et al., 2012; Cabreiro et al., 2013). Supplementation with nicotinamide, which is one form of vitamin B3, shortens the lifespan of budding yeast by decreasing the deacetylase activity of Sir2 (Bitterman et al., 2002). Overall, these studies indicate that the conventional view that vitamins promote health benefits and delay aging should be modified or applied with caution.
How can we explain these differential effects of vitamin supplementation on lifespan? One plausible interpretation is hormesis, which is defined as beneficial effects of low doses of substances that are toxic at higher doses. Thus, hormetic effects of vitamins predict that high doses of vitamins have negative effects on the health and aging, while low doses are beneficial for health (Hayes, 2007). In the same context, although the amount of vitamins that are required for the proper functions of our body is relatively small, deficiency of vitamins causes diseases. The triage theory may help explain the effects of vitamin deficiency on health (Ames, 2006; McCann & Ames, 2009). According to this theory, when a micronutrient is insufficient, nature prioritizes biological functions essential for short-term survival by the expense of nonessential functions. This leads to long-term consequences that may cause age-related diseases. In any case, these possibilities are consistent with the fact that adequate amounts of vitamins are crucial for the management of health.
In comparison with vitamins, the effects of dietary minerals on aging are not as well known. Examples of the beneficial effects of minerals are rare. One example is a study showing that dietary intake of selenium (Se), an antioxidant mineral, significantly reduces DNA breakage and extends the replicative lifespan of cultured adrenocortical cells (Hornsby & Harris, 1987). However, supplementation with high doses of minerals generally decreases organismal lifespan. Supplementation with various doses of selenium (Se), iron (Fe), manganese (Mn), copper (Cu), or zinc (Zn) leads to reduced lifespan in D. melanogaster and C. elegans (Hornsby & Harris, 1987; Wang et al., 2007; Hu et al., 2008; Bahadorani et al., 2010; Helmcke et al., 2010; Bonilla et al., 2012; Selman et al., 2013). Overexpression of metal-responsive transcription factor, MTF-1, rescues the reduced lifespan of flies induced after supplementation with high, millimolar doses of metals (Bahadorani et al., 2010). Thus, excessive amounts of dietary minerals are generally harmful to organisms.
Conclusions
It is well documented that CR increases lifespan in various organisms. However, CR can be difficult to execute for humans because of various reasons. Although drugs that mimic CR have been extensively sought to obtain the benefits of CR without reducing caloric intake, the effects of these drugs on human aging and health are not fully verified (Mouchiroud et al., 2010). Altering the amounts of each individual nutritional component in food is probably less difficult than restricting overall caloric intake, because one may not have to suffer from hunger with proper diet plans. In this review, we discussed findings that each dietary nutritional component, such as carbohydrates, proteins and amino acids, lipids, and vitamins and minerals, influences lifespan in a diverse range of model organisms. These studies raise the possibility that restriction or intake of certain types of nutrients may extend lifespan in humans as well.
There are many remaining challenges ahead in the field. First, although alteration of one nutrient can affect lifespan, this may lead to a change in the intake or processing of other nutrients in the mixture. In this regard, some of the studies on the reduction of a single nutritional component may have been misinterpreted. In addition, recent studies indicate that dietary balance among nutrients has bigger effects on aging than individual components (Lee et al., 2008; Skorupa et al., 2008; Solon-Biet et al., 2014). Indeed, many studies show that protein/nonprotein nutrient ratio rather than amount of proteins or calories plays key roles in the regulation of lifespan (Mair et al., 2005; Lee et al., 2008; Skorupa et al., 2008; Fanson et al., 2009; Bruce et al., 2013). The Nutritional Geometric Framework (NGF) is a state-space approach that represents the effects of the number and the nature of nutrient dimensions on biological responses including lifespan (Fanson et al., 2009; reviewed in Piper et al., 2011; and Tatar et al., 2014). NGF provides new insights into the impact of multiple nutrients on DR relative to each other. So far, this method has mostly been applied for the impact of protein intake relative to carbohydrates. Using this method, in the future, mechanisms by which different compositions of various nutrients, including lipids, amino acids, and vitamins, affect aging can be dissected better. Second, genetic factors that mediate the effects of nutritional components on aging have been mostly focused on insulin/IGF-1 signaling, TOR signaling and sirtuins, but it does not necessarily mean that these factors are the most important factors. Therefore, identification of genetic factors using unbiased methods and systems biology approaches may lead to better mechanistic insights. Third, although studies on human subjects offer invaluable information about the effects of dietary nutritional components on health and aging, more studies on primates and humans are required. For example, the effects of DR on primate longevity are controversial, perhaps due to differences in dietary nutrient composition (Colman et al., 2009, 2014; Mattison et al., 2012). In the future, it will be exciting to combine all these approaches to translate discoveries in model organisms into therapeutic applications.
Acknowledgments
We thank the anonymous reviewers and Lee lab members for helpful comments for improving our manuscript. We apologize to the authors whose papers were not cited in this manuscript because of space limits.
Funding
This research was supported by the National Research Foundation of Korea (NRF) Grant funded by the Korean Government (MSIP) (NRF-2012R1A4A1028200, NRF-2013R1A1A2014754), by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare (HI11C1609) to S-J.L, D.E.J is supported by an NRF Grant (Fostering Core Leaders of the Future Basic Science Program; NRF-2013H1A8A1003751), and W.H. is supported by TJ Park Science Fellowship of POSCO, TJ Park Foundation.
Conflict of interest
The authors declare no conflict of interests.
References
- Allen NE, Appleby PN, Key TJ, Bueno-de-Mesquita HB, Ros MM, Kiemeney LA, Tjonneland A, Roswall N, Overvad K, Weikert S, Boeing H, Chang-Claude J, Teucher B, Panico S, Sacerdote C, Tumino R, Palli D, Sieri S, Peeters P, Quiros JR, Jakszyn P, Molina-Montes E, Chirlaque MD, Ardanaz E, Dorronsoro M, Khaw KT, Wareham N, Ljungberg B, Hallmans G, Ehrnstrom R, Ericson U, Gram IT, Parr CL, Trichopoulou A, Karapetyan T, Dilis V, Clavel-Chapelon F, Boutron-Ruault MC, Fagherrazzi G, Romieu I, Gunter MJ, Riboli E. Macronutrient intake and risk of urothelial cell carcinoma in the European prospective investigation into cancer and nutrition. Int. J. Cancer. 2013;132:635–644. doi: 10.1002/ijc.27643. [DOI] [PubMed] [Google Scholar]
- Ames BN. Low micronutrient intake may accelerate the degenerative diseases of aging through allocation of scarce micronutrients by triage. Proc. Natl Acad. Sci. USA. 2006;103:17589–17594. doi: 10.1073/pnas.0608757103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames BN, Atamna H, Killilea DW. Mineral and vitamin deficiencies can accelerate the mitochondrial decay of aging. Mol. Aspects Med. 2005;26:363–378. doi: 10.1016/j.mam.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Appel LJ, Sacks FM, Carey VJ, Obarzanek E, Swain JF, Miller ER, III, Conlin PR, Erlinger TP, Rosner BA, Laranjo NM, Charleston J, McCarron P, Bishop LM. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA. 2005;294:2455–2464. doi: 10.1001/jama.294.19.2455. [DOI] [PubMed] [Google Scholar]
- Aston LM. Glycaemic index and metabolic disease risk. Proc. Nutr. Soc. 2006;65:125–134. doi: 10.1079/pns2005485. [DOI] [PubMed] [Google Scholar]
- Bahadorani S, Mukai S, Egli D, Hilliker AJ. Overexpression of metal-responsive transcription factor (MTF-1) in Drosophila melanogaster ameliorates life-span reductions associated with oxidative stress and metal toxicity. Neurobiol. Aging. 2010;31:1215–1226. doi: 10.1016/j.neurobiolaging.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Banks AS, Kon N, Knight C, Matsumoto M, Gutierrez-Juarez R, Rossetti L, Gu W, Accili D. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 2008;8:333–341. doi: 10.1016/j.cmet.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay AW, Petocz P, McMillan-Price J, Flood VM, Prvan T, Mitchell P, Brand-Miller JC. Glycemic index, glycemic load, and chronic disease risk – a meta-analysis of observational studies. Am. J. Clin. Nutr. 2008;87:627–637. doi: 10.1093/ajcn/87.3.627. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitterman KJ, Anderson RM, Cohen HY, Latorre-Esteves M, Sinclair DA. Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast sir2 and human SIRT1. J. Biol. Chem. 2002;277:45099–45107. doi: 10.1074/jbc.M205670200. [DOI] [PubMed] [Google Scholar]
- Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297:842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- Bonilla E, Contreras R, Medina-Leendertz S, Mora M, Villalobos V, Bravo Y. Minocycline increases the life span and motor activity and decreases lipid peroxidation in manganese treated Drosophila melanogaster. Toxicology. 2012;294:50–53. doi: 10.1016/j.tox.2012.01.016. [DOI] [PubMed] [Google Scholar]
- Bruce KD, Hoxha S, Carvalho GB, Yamada R, Wang HD, Karayan P, He S, Brummel T, Kapahi P, Ja WW. High carbohydrate-low protein consumption maximizes Drosophila lifespan. Exp. Gerontol. 2013;48:1129–1135. doi: 10.1016/j.exger.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buch S, Melcher C, Bauer M, Katzenberger J, Pankratz MJ. Opposing effects of dietary protein and sugar regulate a transcriptional target of Drosophila insulin-like peptide signaling. Cell Metab. 2008;7:321–332. doi: 10.1016/j.cmet.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Cabreiro F, Au C, Leung KY, Vergara-Irigaray N, Cocheme HM, Noori T, Weinkove D, Schuster E, Greene ND, Gems D. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell. 2013;153:228–239. doi: 10.1016/j.cell.2013.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Pearman AT, Zimmerman GA, McIntyre TM, Prescott SM. Intracellular unesterified arachidonic acid signals apoptosis. Proc. Natl Acad. Sci. 2000;97:11280–11285. doi: 10.1073/pnas.200367597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey JR, Harshman LG, Liedo P, Muller HG, Wang JL, Zhang Z. Longevity-fertility trade-offs in the tephritid fruit fly, Anastrepha ludens, across dietary-restriction gradients. Aging Cell. 2008;7:470–477. doi: 10.1111/j.1474-9726.2008.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro P, Gomez J, Sanchez I, Garcia R, Lopez-Torres M, Naudi A, Portero-Otin M, Pamplona R, Barja G. Effect of 40% restriction of dietary amino acids (except methionine) on mitochondrial oxidative stress and biogenesis, AIF and SIRT1 in rat liver. Biogerontology. 2009;10:579–592. doi: 10.1007/s10522-008-9200-4. [DOI] [PubMed] [Google Scholar]
- Chalkiadaki A, Guarente L. High-fat diet triggers inflammation-induced cleavage of SIRT1 in adipose tissue to promote metabolic dysfunction. Cell Metab. 2012;16:180–188. doi: 10.1016/j.cmet.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JB, Sun J, Jazwinski SM. Prolongation of the yeast life span by the v-Ha-RAS oncogene. Mol. Microbiol. 1990;4:2081–2086. doi: 10.1111/j.1365-2958.1990.tb00568.x. [DOI] [PubMed] [Google Scholar]
- Choi SS. High glucose diets shorten lifespan of Caenorhabditis elegans via ectopic apoptosis induction. Nutr. Res. Pract. 2011;5:214–218. doi: 10.4162/nrp.2011.5.3.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Beasley TM, Kemnitz JW, Johnson SC, Weindruch R, Anderson RM. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat. Commun. 2014;5:3557. doi: 10.1038/ncomms4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui R, Gao M, Qu S, Liu D. Overexpression of superoxide dismutase 3 gene blocks high-fat diet-induced obesity, fatty liver and insulin resistance. Gene Ther. 2014;21:840–848. doi: 10.1038/gt.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallaire A, Garand C, Paquel ER, Mitchell SJ, de Cabo R, Simard MJ, Lebel M. Down regulation of miR-124 in both Werner syndrome DNA helicase mutant mice and mutant Caenorhabditis elegans wrn-1 reveals the importance of this microRNA in accelerated aging. Aging. 2012;4:636–647. doi: 10.18632/aging.100489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das UN. Folic acid and polyunsaturated fatty acids improve cognitive function and prevent depression, dementia, and Alzheimer's disease—But how and why? Prostaglandins Leukot. Essent. Fatty Acids. 2008;78:11–19. doi: 10.1016/j.plefa.2007.10.006. [DOI] [PubMed] [Google Scholar]
- De Marte ML, Enesco HE. Influence of low tryptophan diet on survival and organ growth in mice. Mech. Ageing Dev. 1986;36:161–171. doi: 10.1016/0047-6374(86)90017-5. [DOI] [PubMed] [Google Scholar]
- Denzel MS, Storm NJ, Gutschmidt A, Baddi R, Hinze Y, Jarosch E, Sommer T, Hoppe T, Antebi A. Hexosamine pathway metabolites enhance protein quality control and prolong life. Cell. 2014;156:1167–1178. doi: 10.1016/j.cell.2014.01.061. [DOI] [PubMed] [Google Scholar]
- Diaz-Albiter H, Mitford R, Genta FA, Sant'Anna MRV, Dillon RJ. Reactive oxygen species scavenging by catalase is important for female Lutzomyia longipalpis fecundity and mortality. PLoS One. 2011;6:e17486. doi: 10.1371/journal.pone.0017486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon LJ, Flask CA, Papouchado BG, Feldstein AE, Nagy LE. Caspase-1 as a central regulator of high fat diet-induced non-alcoholic steatohepatitis. PLoS One. 2013;8:e56100. doi: 10.1371/journal.pone.0056100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong JS, Qiu HF, Garcia-Barrio M, Anderson J, Hinnebusch AG. Uncharged tRNA activates GCN2 by displacing the protein kinase moiety from a bipartite tRNA-Binding domain. Mol. Cell. 2000;6:269–279. doi: 10.1016/s1097-2765(00)00028-9. [DOI] [PubMed] [Google Scholar]
- Edwards CB, Copes N, Brito AG, Canfield J, Bradshaw PC. Malate and fumarate extend lifespan in Caenorhabditis elegans. PLoS One. 2013;8:e58345. doi: 10.1371/journal.pone.0058345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanson BG, Weldon CW, Perez-Staples D, Simpson SJ, Taylor PW. Nutrients, not caloric restriction, extend lifespan in Queensland fruit flies (Bactrocera tryoni. Aging Cell. 2009;8:514–523. doi: 10.1111/j.1474-9726.2009.00497.x. [DOI] [PubMed] [Google Scholar]
- Feige JN, Lagouge M, Canto C, Strehle A, Houten SM, Milne JC, Lambert PD, Mataki C, Elliott PJ, Auwerx J. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab. 2008;8:347–358. doi: 10.1016/j.cmet.2008.08.017. [DOI] [PubMed] [Google Scholar]
- Feng Y, Williams BG, Koumanov F, Wolstenholme AJ, Holman GD. FGT-1 is the major glucose transporter in C. elegans and is central to aging pathways. Biochem. J. 2013;456:219–229. doi: 10.1042/BJ20131101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez ML. Dietary cholesterol provided by eggs and plasma lipoproteins in healthy populations. Curr. Opin. Clin. Nutr. Metab. Care. 2006;9:8–12. doi: 10.1097/01.mco.0000171152.51034.bf. [DOI] [PubMed] [Google Scholar]
- Filhoulaud G, Guilmeau S, Dentin R, Girard J, Postic C. Novel insights into ChREBP regulation and function. Trends Endocrinol. Metab. 2013;24:257–268. doi: 10.1016/j.tem.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Foster GD, Wyatt HR, Hill JO, McGuckin BG, Brill C, Mohammed BS, Szapary PO, Rader DJ, Edman JS, Klein S. A randomized trial of a low-carbohydrate diet for obesity. N. Engl. J. Med. 2003;348:2082–2090. doi: 10.1056/NEJMoa022207. [DOI] [PubMed] [Google Scholar]
- Gallinetti J, Harputlugil E, Mitchell JR. Amino acid sensing in dietary-restriction-mediated longevity: roles of signal-transducing kinases GCN2 and TOR. Biochem. J. 2013;449:1–10. doi: 10.1042/BJ20121098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg SK, Mahajan S. Effect of ascorbic acid on longevity, catalase and lipid peroxidation in Callosobruchus maculatus F. Age. 1993;16:87–92. [Google Scholar]
- Gilani GS, Ratnayake WMN, Peace RW, Mueller R. Effects of supplemental cystine or methionine on growth and lifespan of stroke-prone spontaneously hypertensive rats. Br. J. Nutr. 2006;95:443–447. doi: 10.1079/bjn20051712. [DOI] [PubMed] [Google Scholar]
- Gomez-Cabrera MC, Domenech E, Romagnoli M, Arduini A, Borras C, Pallardo FV, Sastre J, Vina J. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am. J. Clin. Nutr. 2008;87:142–149. doi: 10.1093/ajcn/87.1.142. [DOI] [PubMed] [Google Scholar]
- Grandison RC, Piper MDW, Partridge L. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature. 2009;462:1061–U1121. doi: 10.1038/nature08619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grygiel-Gorniak B. Peroxisome proliferator-activated receptors and their ligands: nutritional and clinical implications – a review. Nutr. J. 2014;13:17. doi: 10.1186/1475-2891-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigis MC, Guarente LP. Mammalian sirtuins–emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20:2913–2921. doi: 10.1101/gad.1467506. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. Oxford: Oxford University Press; 1999. [Google Scholar]
- Harrington LA, Harley CB. Effect of vitamin E on lifespan and reproduction in Caenorhabditis elegans. Mech. Ageing Dev. 1988;43:71–78. doi: 10.1016/0047-6374(88)90098-x. [DOI] [PubMed] [Google Scholar]
- Hayes DP. Nutritional hormesis. Eur. J. Clin. Nutr. 2007;61:147–159. doi: 10.1038/sj.ejcn.1602507. [DOI] [PubMed] [Google Scholar]
- Heidler T, Hartwig K, Daniel H, Wenzel U. Caenorhabditis elegans lifespan extension caused by treatment with an orally active ROS-generator is dependent on DAF-16 and SIR-2.1. Biogerontology. 2010;11:183–195. doi: 10.1007/s10522-009-9239-x. [DOI] [PubMed] [Google Scholar]
- Helmcke KJ, Avila DS, Aschner M. Utility of Caenorhabditis elegans in high throughput neurotoxicological research. Neurotoxicol. Teratol. 2010;32:62–67. doi: 10.1016/j.ntt.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Honda H, Qureshi AR, Axelsson J, Heimburger O, Suliman ME, Barany P, Stenvinkel P, Lindholm B. Obese sarcopenia in patients with end-stage renal disease is associated with inflammation and increased mortality. Am. J. Clin. Nutr. 2007;86:633–638. doi: 10.1093/ajcn/86.3.633. [DOI] [PubMed] [Google Scholar]
- Honda Y, Tanaka M, Honda S. Trehalose extends longevity in the nematode Caenorhabditis elegans. Aging Cell. 2010;9:558–569. doi: 10.1111/j.1474-9726.2010.00582.x. [DOI] [PubMed] [Google Scholar]
- Hornsby PJ, Harris SE. Oxidative damage to DNA and replicative lifespan in cultured adrenocortical cells. Exp. Cell Res. 1987;168:203–217. doi: 10.1016/0014-4827(87)90429-0. [DOI] [PubMed] [Google Scholar]
- Hu YO, Wang Y, Ye BP, Wang DY. Phenotypic and behavioral defects induced by iron exposure can be transferred to progeny in Caenorhabditis elegans. Biomed. Environ. Sci. 2008;21:467–473. doi: 10.1016/S0895-3988(09)60004-0. [DOI] [PubMed] [Google Scholar]
- Hulbert AJ. Metabolism and longevity: is there a role for membrane fatty acids? Integr. Comp. Biol. 2010;50:808–817. doi: 10.1093/icb/icq007. [DOI] [PubMed] [Google Scholar]
- Hulbert AJ, Beard LA, Grigg GC. The exceptional longevity of an egg-laying mammal, the short-beaked echidna (Tachyglossus aculeatus) is associated with peroxidation-resistant membrane composition. Exp. Gerontol. 2008;43:729–733. doi: 10.1016/j.exger.2008.05.015. [DOI] [PubMed] [Google Scholar]
- Iwasaki K, Gleiser CA, Masoro EJ, McMahan CA, Seo EJ, Yu BP. The influence of dietary protein source on longevity and age-related disease processes of Fischer rats. J. Gerontol. 1988a;43:B5–12. doi: 10.1093/geronj/43.1.b5. [DOI] [PubMed] [Google Scholar]
- Iwasaki K, Gleiser CA, Masoro EJ, McMahan CA, Seo EJ, Yu BP. Influence of the restriction of individual dietary components on longevity and age-related disease of Fischer rats: the fat component and the mineral component. J. Gerontol. 1988b;43:B13–21. doi: 10.1093/geronj/43.1.b13. [DOI] [PubMed] [Google Scholar]
- Jafarirad S, Siassi F, Harirchian MH, Amani R, Bitarafan S, Saboor-Yaraghi A. The effect of vitamin a supplementation on biochemical parameters in multiple sclerosis patients. Iran. Red Crescent Med. J. 2013;15:194–198. doi: 10.5812/ircmj.3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins DJ, Wolever TM, Taylor RH, Barker H, Fielden H, Baldwin JM, Bowling AC, Newman HC, Jenkins AL, Goff DV. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am. J. Clin. Nutr. 1981;34:362–366. doi: 10.1093/ajcn/34.3.362. [DOI] [PubMed] [Google Scholar]
- Jeon TI, Osborne TF. SREBPs: metabolic integrators in physiology and metabolism. Trends Endocrinol. Metab. 2012;23:65–72. doi: 10.1016/j.tem.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr. Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassi E, Papavassiliou AG. Could glucose be a proaging factor? J. Cell Mol. Med. 2008;12:1194–1198. doi: 10.1111/j.1582-4934.2008.00329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon CJ. The genetics of ageing. Nature. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- Kitaoka S, Morielli AD, Zhao FQ. FGT-1 is a mammalian GLUT2-like facilitative glucose transporter in Caenorhabditis elegans whose malfunction induces fat accumulation in intestinal cells. PLoS One. 2013;8:e68475. doi: 10.1371/journal.pone.0068475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Laxman S, Sutter BM, Wu X, Kumar S, Guo X, Trudgian DC, Mirzaei H, Tu BP. Sulfur amino acids regulate translational capacity and metabolic homeostasis through modulation of tRNA thiolation. Cell. 2013;154:416–429. doi: 10.1016/j.cell.2013.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KP, Simpson SJ, Clissold FJ, Brooks R, Ballard JW, Taylor PW, Soran N, Raubenheimer D. Lifespan and reproduction in Drosophila: new insights from nutritional geometry. Proc. Natl Acad. Sci. USA. 2008;105:2498–2503. doi: 10.1073/pnas.0710787105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Murphy CT, Kenyon C. Glucose shortens the life span of C. elegans by downregulating DAF-16/FOXO activity and aquaporin gene expression. Cell Metab. 2009;10:379–391. doi: 10.1016/j.cmet.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Hwang AB, Kenyon C. Inhibition of respiration extends C. elegans life span via reactive oxygen species that increase HIF-1 activity. Curr. Biol. 2010;20:2131–2136. doi: 10.1016/j.cub.2010.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BC, Kaya A, Ma S, Kim G, Gerashchenko MV, Yim SH, Hu Z, Harshman LG, Gladyshev VN. Methionine restriction extends lifespan of Drosophila melanogaster under conditions of low amino-acid status. Nat. Commun. 2014;5:3592. doi: 10.1038/ncomms4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesperance ML, Olivotto IA, Forde N, Zhao Y, Speers C, Foster H, Tsao M, MacPherson N, Hoffer A. Mega-dose vitamins and minerals in the treatment of non-metastatic breast cancer: an historical cohort study. Breast Cancer Res. Treat. 2002;76:137–143. doi: 10.1023/a:1020552501345. [DOI] [PubMed] [Google Scholar]
- Levine ME, Suarez JA, Brandhorst S, Balasubramanian P, Cheng CW, Madia F, Fontana L, Mirisola MG, Guevara-Aguirre J, Wan J, Passarino G, Kennedy BK, Wei M, Cohen P, Crimmins EM, Longo VD. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014;19:407–417. doi: 10.1016/j.cmet.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao FH, Shieh MJ, Yang SC, Lin SH, Chien YW. Effectiveness of a soy-based compared with a traditional low-calorie diet on weight loss and lipid levels in overweight adults. Nutrition. 2007;23:551–556. doi: 10.1016/j.nut.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Lin Y, Bolca S, Vandevijvere S, De Vriese S, Mouratidou T, De Neve M, Polet A, Van Oyen H, Van Camp J, De Backer G, De Henauw S, Huybrechts I. Plant and animal protein intake and its association with overweight and obesity among the Belgian population. Br. J. Nutr. 2011;105:1106–1116. doi: 10.1017/S0007114510004642. [DOI] [PubMed] [Google Scholar]
- Lomb DJ, Laurent G, Haigis MC. Sirtuins regulate key aspects of lipid metabolism. Biochim. Biophys. Acta. 2010;1804:1652–1657. doi: 10.1016/j.bbapap.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Maeda H, Gleiser CA, Masoro EJ, Murata I, McMahan CA, Yu BP. Nutritional influences on aging of Fischer 344 rats: II. Pathology. J. Gerontol. 1985;40:671–688. doi: 10.1093/geronj/40.6.671. [DOI] [PubMed] [Google Scholar]
- Mair W, Piper MD, Partridge L. Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biol. 2005;3:e223. doi: 10.1371/journal.pbio.0030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maklakov AA, Simpson SJ, Zajitschek F, Hall MD, Dessmann J, Clissold F, Raubenheimer D, Bonduriansky R, Brooks RC. Sex-specific fitness effects of nutrient intake on reproduction and lifespan. Curr. Biol. 2008;18:1062–1066. doi: 10.1016/j.cub.2008.06.059. [DOI] [PubMed] [Google Scholar]
- Masoro EJ. Nutrition and aging–a current assessment. J. Nutr. 1985;115:842–848. doi: 10.1093/jn/115.7.842. [DOI] [PubMed] [Google Scholar]
- Masoro EJ. Caloric Restriction: A Key to Understanding and Modulating Aging. Amsterdam; Boston: Elsevier; 2002. [Google Scholar]
- Masoro EJ, Iwasaki K, Gleiser CA, McMahan CA, Seo EJ, Yu BP. Dietary modulation of the progression of nephropathy in aging rats: an evaluation of the importance of protein. Am. J. Clin. Nutr. 1989;49:1217–1227. doi: 10.1093/ajcn/49.6.1217. [DOI] [PubMed] [Google Scholar]
- Massie HR, Shumway ME, Whitney SJP, Sternick SM, Aiello VR. Ascorbic acid in Drosophila and changes during aging. Exp. Gerontol. 1991;26:487–494. doi: 10.1016/0531-5565(91)90037-m. [DOI] [PubMed] [Google Scholar]
- Massie HR, Aiello VR, Williams TR. Influence of photosensitizers and light on the life span of Drosophila. Mech. Ageing Dev. 1993;68:175–182. doi: 10.1016/0047-6374(93)90149-l. [DOI] [PubMed] [Google Scholar]
- Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M, Barnard D, Ward WF, Qi W, Ingram DK, de Cabo R. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489:318–321. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann JC, Ames BN. Vitamin K, an example of triage theory: is micronutrient inadequacy linked to diseases of aging? Am. J. Clin. Nutr. 2009;90:889–907. doi: 10.3945/ajcn.2009.27930. [DOI] [PubMed] [Google Scholar]
- McCarty MF, Barroso-Aranda J, Contreras F. The low-methionine content of vegan diets may make methionine restriction feasible as a life extension strategy. Med. Hypotheses. 2009;72:125–128. doi: 10.1016/j.mehy.2008.07.044. [DOI] [PubMed] [Google Scholar]
- Meissner B, Boll M, Daniel H, Baumeister R. Deletion of the intestinal peptide transporter affects insulin and TOR signaling in Caenorhabditis elegans. J. Biol. Chem. 2004;279:36739–36745. doi: 10.1074/jbc.M403415200. [DOI] [PubMed] [Google Scholar]
- Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am. J. Clin. Nutr. 2003;77:1146–1155. doi: 10.1093/ajcn/77.5.1146. [DOI] [PubMed] [Google Scholar]
- Miller RA, Buehner G, Chang Y, Harper JM, Sigler R, Smith-Wheelock M. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell. 2005;4:119–125. doi: 10.1111/j.1474-9726.2005.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min KJ, Tatar M. Restriction of amino acids extends lifespan in Drosophila melanogaster. Mech. Ageing Dev. 2006;127:643–646. doi: 10.1016/j.mad.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Minor RK, Baur JA, Gomes AP, Ward TM, Csiszar A, Mercken EM, Abdelmohsen K, Shin YK, Canto C, Scheibye-Knudsen M, Krawczyk M, Irusta PM, Martin-Montalvo A, Hubbard BP, Zhang Y, Lehrmann E, White AA, Price NL, Swindell WR, Pearson KJ, Becker KG, Bohr VA, Gorospe M, Egan JM, Talan MI, Auwerx J, Westphal CH, Ellis JL, Ungvari Z, Vlasuk GP, Elliott PJ, Sinclair DA, de Cabo R. SRT1720 improves survival and healthspan of obese mice. Sci. Rep. 2011;1:70. doi: 10.1038/srep00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miquel J, Fleming J, Economos AC. Antioxidants, metabolic rate and aging in Drosophila. Arch. Gerontol. Geriatr. 1982;1:159–165. doi: 10.1016/0167-4943(82)90016-4. [DOI] [PubMed] [Google Scholar]
- Mitchell TW, Buffenstein R, Hulbert AJ. Membrane lipid composition may contribute to exceptional longevity of the naked mole-rat (Heterocephalus glaber): a comparative study using shotgun lipidomics. Exp. Gerontol. 2007;42:1053–1062. doi: 10.1016/j.exger.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Mortuza R, Chen S, Feng B, Sen S, Chakrabarti S. High glucose induced alteration of SIRTs in endothelial cells causes rapid aging in a p300 and FOXO regulated pathway. PLoS One. 2013;8:e54514. doi: 10.1371/journal.pone.0054514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouchiroud L, Molin L, Dalliere N, Solari F. Life span extension by resveratrol, rapamycin, and metformin: the promise of dietary restriction mimetics for an healthy aging. BioFactors. 2010;36:377–382. doi: 10.1002/biof.127. [DOI] [PubMed] [Google Scholar]
- Mouchiroud L, Molin L, Kasturi P, Triba MN, Dumas ME, Wilson MC, Halestrap AP, Roussel D, Masse I, Dalliere N, Segalat L, Billaud M, Solari F. Pyruvate imbalance mediates metabolic reprogramming and mimics lifespan extension by dietary restriction in Caenorhabditis elegans. Aging Cell. 2011;10:39–54. doi: 10.1111/j.1474-9726.2010.00640.x. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D. Low-fat diet and cardiovascular disease. JAMA. 2006a;296:279–280. doi: 10.1001/jama.296.3.279-b. ; author reply 280–271. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D. Trans fatty acids – effects on systemic inflammation and endothelial function. Atheroscler. Suppl. 2006b;7:29–32. doi: 10.1016/j.atherosclerosissup.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Pischon T, Hankinson SE, Rifai N, Joshipura K, Willett WC, Rimm EB. Dietary intake of trans fatty acids and systemic inflammation in women. Am. J. Clin. Nutr. 2004;79:606–612. doi: 10.1093/ajcn/79.4.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro A, Gomez C, Sanchez-Pino MJ, Gonzalez H, Bandez MJ, Boveris AD, Boveris A. Vitamin E at high doses improves survival, neurological performance, and brain mitochondrial function in aging male mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;289:R1392–1399. doi: 10.1152/ajpregu.00834.2004. [DOI] [PubMed] [Google Scholar]
- O'Rourke EJ, Kuballa P, Xavier R, Ruvkun G. 6 Polyunsaturated fatty acids extend life span through the activation of autophagy. Genes Dev. 2013;27:429–440. doi: 10.1101/gad.205294.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacholec M, Bleasdale JE, Chrunyk B, Cunningham D, Flynn D, Garofalo RS, Griffith D, Griffor M, Loulakis P, Pabst B, Qiu X, Stockman B, Thanabal V, Varghese A, Ward J, Withka J, Ahn K. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J. Biol. Chem. 2010;285:8340–8351. doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamplona R, Portero-Otin M, Riba D, Ruiz C, Prat J, Bellmunt MJ, Barja G. Mitochondrial membrane peroxidizability index is inversely related to maximum life span in mammals. J. Lipid Res. 1998;39:1989–1994. [PubMed] [Google Scholar]
- Peng W, Robertson L, Gallinetti J, Mejia P, Vose S, Charlip A, Chu T, Mitchell JR. Surgical stress resistance induced by single amino acid deprivation requires Gcn2 in mice. Sci. Transl. Med. 2012;4:118ra111. doi: 10.1126/scitranslmed.3002629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschop MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc. Natl Acad. Sci. USA. 2008;105:9793–9798. doi: 10.1073/pnas.0802917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper MD, Mair W, Partridge L. Counting the calories: the role of specific nutrients in extension of life span by food restriction. J. Gerontol. A Biol. Sci. Med. Sci. 2005;60:549–555. doi: 10.1093/gerona/60.5.549. [DOI] [PubMed] [Google Scholar]
- Piper MD, Partridge L, Raubenheimer D, Simpson SJ. Dietary restriction and aging: a unifying perspective. Cell Metab. 2011;14:154–160. doi: 10.1016/j.cmet.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss HG, Echard B, Clouatre D, Bagchi D, Perricone NV. Niacin-bound chromium increases life span in Zucker Fatty Rats. J. Inorg. Biochem. 2011;105:1344–1349. doi: 10.1016/j.jinorgbio.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Price NL, Gomes AP, Ling AJ, Duarte FV, Martin-Montalvo A, North BJ, Agarwal B, Ye L, Ramadori G, Teodoro JS, Hubbard BP, Varela AT, Davis JG, Varamini B, Hafner A, Moaddel R, Rolo AP, Coppari R, Palmeira CM, de Cabo R, Baur JA, Sinclair DA. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15:675–690. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puca AA, Andrew P, Novelli V, Anselmi CV, Somalvico F, Cirillo NA, Chatgilialoglu C, Ferreri C. Fatty acid profile of erythrocyte membranes as possible biomarker of longevity. Rejuvenation Res. 2008;11:63–72. doi: 10.1089/rej.2007.0566. [DOI] [PubMed] [Google Scholar]
- Riserus U, Willett WC, Hu FB. Dietary fats and prevention of type 2 diabetes. Prog. Lipid Res. 2009;48:44–51. doi: 10.1016/j.plipres.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristow M, Zarse K, Oberbach A, Kloting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Bluher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc. Natl Acad. Sci. USA. 2009;106:8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosedale R, Westman EC, Konhilas JP. Clinical experience of a diet designed to reduce aging. J. Appl. Res. 2009;9:159–165. [PMC free article] [PubMed] [Google Scholar]
- Roux AE, Leroux A, Alaamery MA, Hoffman CS, Chartrand P, Ferbeyre G, Rokeach LA. Pro-aging effects of glucose signaling through a G protein-coupled glucose receptor in fission yeast. PLoS Genet. 2009;5:e1000408. doi: 10.1371/journal.pgen.1000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Roman I, Gomez A, Perez I, Sanchez C, Suarez H, Naudi A, Jove M, Lopez-Torres M, Pamplona R, Barja G. Effects of aging and methionine restriction applied at old age on ROS generation and oxidative damage in rat liver mitochondria. Biogerontology. 2012;13:399–411. doi: 10.1007/s10522-012-9384-5. [DOI] [PubMed] [Google Scholar]
- Sanz A, Caro P, Barja G. Protein restriction without strong caloric restriction decreases mitochondrial oxygen radical production and oxidative DNA damage in rat liver. J. Bioenerg. Biomembr. 2004;36:545–552. doi: 10.1007/s10863-004-9001-7. [DOI] [PubMed] [Google Scholar]
- Sanz A, Caro P, Ayala V, Portero-Otin M, Pamplona R, Barja G. Methionine restriction decreases mitochondrial oxygen radical generation and leak as well as oxidative damage to mitochondrial DNA and proteins. FASEB J. 2006;20:1064–1073. doi: 10.1096/fj.05-5568com. [DOI] [PubMed] [Google Scholar]
- Sawada M, Enesco HE. Vitamin E extends lifespan in the short-lived rotifer Asplanchna brightwelli. Exp. Gerontol. 1984;19:179–183. doi: 10.1016/0531-5565(84)90036-6. [DOI] [PubMed] [Google Scholar]
- Schlotterer A, Kukudov G, Bozorgmehr F, Hutter H, Du X, Oikonomou D, Ibrahim Y, Pfisterer F, Rabbani N, Thornalley P, Sayed A, Fleming T, Humpert P, Schwenger V, Zeier M, Hamann A, Stern D, Brownlee M, Bierhaus A, Nawroth P, Morcos M. C. elegans as model for the study of high glucose- mediated life span reduction. Diabetes. 2009;58:2450–2456. doi: 10.2337/db09-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeisser K, Mansfeld J, Kuhlow D, Weimer S, Priebe S, Heiland I, Birringer M, Groth M, Segref A, Kanfi Y, Price NL, Schmeisser S, Schuster S, Pfeiffer AF, Guthke R, Platzer M, Hoppe T, Cohen HY, Zarse K, Sinclair DA, Ristow M. Role of sirtuins in lifespan regulation is linked to methylation of nicotinamide. Nat. Chem. Biol. 2013;9:693–700. doi: 10.1038/nchembio.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrager MA, Metter EJ, Simonsick E, Ble A, Bandinelli S, Lauretani F, Ferrucci L. Sarcopenic obesity and inflammation in the InCHIANTI study. J. Appl. Physiol. (1985) 2007;102:919–925. doi: 10.1152/japplphysiol.00627.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, Ristow M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007;6:280–293. doi: 10.1016/j.cmet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Segall PE, Timiras PS. Patho-physiologic findings after chronic tryptophan deficiency in rats: a model for delayed growth and aging. Mech. Ageing Dev. 1976;5:109–124. doi: 10.1016/0047-6374(76)90012-9. [DOI] [PubMed] [Google Scholar]
- Selman C, McLaren JS, Collins AR, Duthie GG, Speakman JR. Deleterious consequences of antioxidant supplementation on lifespan in a wild-derived mammal. Biol. Lett. 2013;9:20130432. doi: 10.1098/rsbl.2013.0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am. J. Clin. Nutr. 2010;91:535–546. doi: 10.3945/ajcn.2009.27725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorupa DA, Dervisefendic A, Zwiener J, Pletcher SD. Dietary composition specifies consumption, obesity, and lifespan in Drosophila melanogaster. Aging Cell. 2008;7:478–490. doi: 10.1111/j.1474-9726.2008.00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solon-Biet SM, McMahon AC, Ballard JW, Ruohonen K, Wu LE, Cogger VC, Warren A, Huang X, Pichaud N, Melvin RG, Gokarn R, Khalil M, Turner N, Cooney GJ, Sinclair DA, Raubenheimer D, Le Couteur DG, Simpson SJ. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab. 2014;19:418–430. doi: 10.1016/j.cmet.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers LK, Fielding BA, Bradshaw HA, Ilic V, Beysen C, Clark ML, Moore NR, Frayn KN. Substituting dietary saturated fat with polyunsaturated fat changes abdominal fat distribution and improves insulin sensitivity. Diabetologia. 2002;45:369–377. doi: 10.1007/s00125-001-0768-3. [DOI] [PubMed] [Google Scholar]
- Sun L, Akha AAS, Miller RA, Harper JM. Life-span extension in mice by preweaning food restriction and by methionine restriction in middle age. J. Gerontol. A Biol. Sci. Med. Sci. 2009;64:711–722. doi: 10.1093/gerona/glp051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K, Xiang L, Ishihara S, Matsuura A, Sakagami Y, Qi J. Anti-aging effects of hesperidin on Saccharomyces cerevisiae via inhibition of reactive oxygen species and UTH1 gene expression. Biosci. Biotechnol. Biochem. 2012;76:640–645. doi: 10.1271/bbb.110535. [DOI] [PubMed] [Google Scholar]
- Sutter BM, Wu X, Laxman S, Tu BP. Methionine inhibits autophagy and promotes growth by inducing the SAM-responsive methylation of PP2A. Cell. 2013;154:403–415. doi: 10.1016/j.cell.2013.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar M, Post S, Yu K. Nutrient control of Drosophila longevity. Trends Endocrinol. Metab. 2014;25:509–517. doi: 10.1016/j.tem.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevisol ET, Panek AD, Mannarino SC, Eleutherio EC. The effect of trehalose on the fermentation performance of aged cells of Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2011;90:697–704. doi: 10.1007/s00253-010-3053-x. [DOI] [PubMed] [Google Scholar]
- Virk B, Correia G, Dixon DP, Feyst I, Jia J, Oberleitner N, Briggs Z, Hodge E, Edwards R, Ward J, Gems D, Weinkove D. Excessive folate synthesis limits lifespan in the C. elegansE. coli aging model. BMC Biol. 2012;10:67. doi: 10.1186/1741-7007-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Shen L, Wang Y. The phenotypic and behavioral defects can be transferred from zinc-exposed nematodes to their progeny. Environ. Toxicol. Pharmacol. 2007;24:223–230. doi: 10.1016/j.etap.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Ward NC, Wu JH, Clarke MW, Puddey IB, Burke V, Croft KD, Hodgson JM. The effect of vitamin E on blood pressure in individuals with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. J. Hypertens. 2007;25:227–234. doi: 10.1097/01.hjh.0000254373.96111.43. [DOI] [PubMed] [Google Scholar]
- Weinberger M, Mesquita A, Caroll T, Marks L, Yang H, Zhang Z, Ludovico P, Burhans WC. Growth signaling promotes chronological aging in budding yeast by inducing superoxide anions that inhibit quiescence. Aging. 2010;2:709–726. doi: 10.18632/aging.100215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weindruch R, Walford RL. The Retardation of Aging and Disease by Dietary Restriction. Springfield, IL, USA: C.C. Thomas; 1988. [Google Scholar]
- Wek SA, Zhu S, Wek RC. The histidyl-tRNA synthetase-related sequence in the eIF-2 alpha protein kinase GCN2 interacts with tRNA and is required for activation in response to starvation for different amino acids. Mol. Cell. Biol. 1995;15:4497–4506. doi: 10.1128/mcb.15.8.4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welton RM, Hoffman CS. Glucose monitoring in fission yeast via the Gpa2 galpha, the git5 Gbeta and the git3 putative glucose receptor. Genetics. 2000;156:513–521. doi: 10.1093/genetics/156.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Hekimi S. A mitochondrial superoxide signal triggers increased longevity in Caenorhabditis elegans. PLoS Biol. 2010;8:e1000556. doi: 10.1371/journal.pbio.1000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu BP, Masoro EJ, McMahan CA. Nutritional influences on aging of Fischer 344 rats: I. Physical, metabolic, and longevity characteristics. J. Gerontol. 1985;40:657–670. doi: 10.1093/geronj/40.6.657. [DOI] [PubMed] [Google Scholar]
- Zadra G, Priolo C, Patnaik A, Loda M. New strategies in prostate cancer: targeting lipogenic pathways and the energy sensor AMPK. Clin. Cancer Res. 2010;16:3322–3328. doi: 10.1158/1078-0432.CCR-09-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajitschek F, Zajitschek SR, Friberg U, Maklakov AA. Interactive effects of sex, social environment, dietary restriction, and methionine on survival and reproduction in fruit flies. Age. 2013;35:1193–1204. doi: 10.1007/s11357-012-9445-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Cui S, Bai X, Zhuo L, Sun X, Hong Q, Fu B, Wang J, Chen X, Cai G. SIRT3 overexpression antagonizes high glucose accelerated cellular senescence in human diploid fibroblasts via the SIRT3-FOXO1 signaling pathway. Age. 2013;35:2237–2253. doi: 10.1007/s11357-013-9520-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman JA, Malloy V, Krajcik R, Orentreich N. Nutritional control of aging. Exp. Gerontol. 2003;38:47–52. doi: 10.1016/s0531-5565(02)00149-3. [DOI] [PubMed] [Google Scholar]


