Abstract
Subarachnoid hemorrhage may be complicated by cerebral ischemia which, though reversible initially, can progress to an irreversible neurological deficit. 31P magnetic resonance spectroscopy, which can determine intracellular pH and thus detect areas of ischemia noninvasively, was applied to 10 patients on 30 occasions, at various times after subarachnoid hemorrhage. In 5 of them, there were focal areas of the brain in which the intracellular pH was reduced to < 6.8 compared with the normal range of 7.05 +/- 0.05. Consciousness was impaired in 4 of these patients. Repeat studies in these 4 patients showed that intracellular pH remained abnormally low for several days but eventually returned toward normal. The return of intracellular pH to normal paralleled an improvement in clinical condition in each case. In the fifth patient with lowered regions of intracellular pH, there had been an impaired level of consciousness and a transient focal deficit prior to the single study. In the other 5 patients there were no areas of reduced pHi even though in 3 of them there was intraventricular or cisternal blood shown on brain computerized tomography. In 2 of these 3 patients there were no abnormal neurological signs at the time of the magnetic resonance study. The third patient had a dense and persistent hemiparesis. The remaining two patients had no abnormal neurological signs at any stage. We suggest that the areas of acidosis may reflect ischemia which is potentially reversible. Since the technique is noninvasive, sequential 31P magnetic resonance spectroscopy of the brain offers a method of detecting cerebral ischemia and, more importantly, of assessing methods of treatment.
Full text
PDF
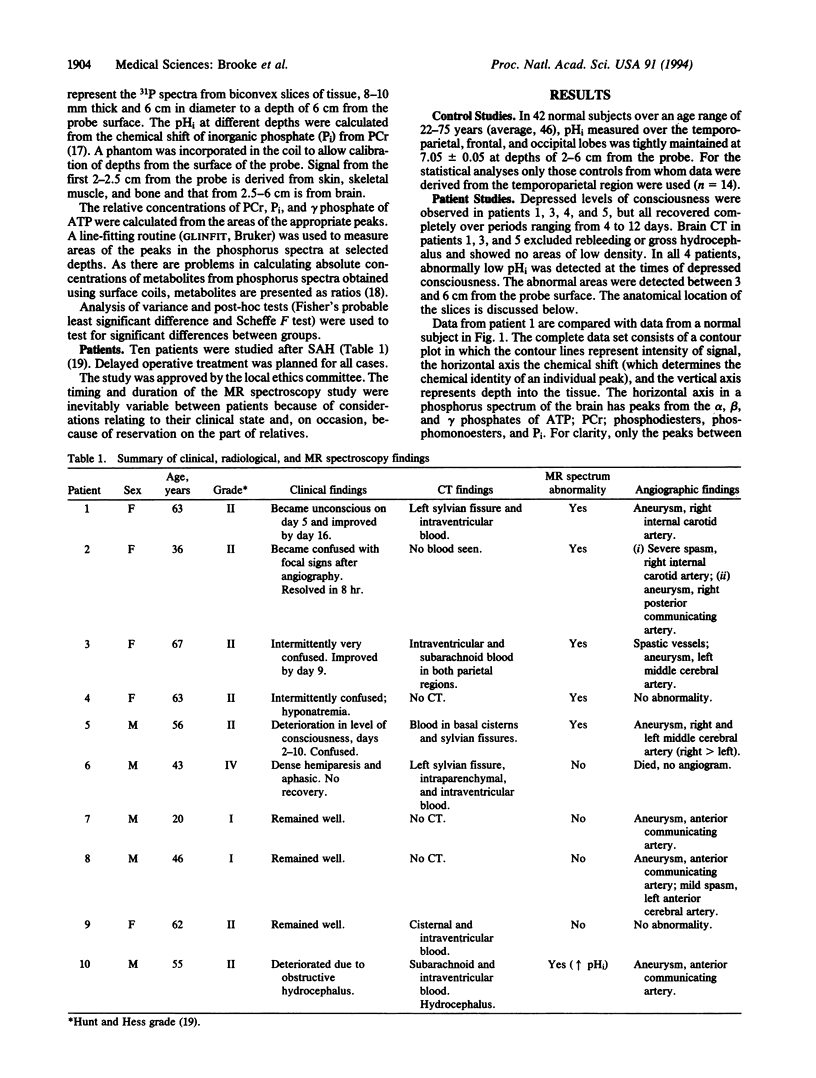
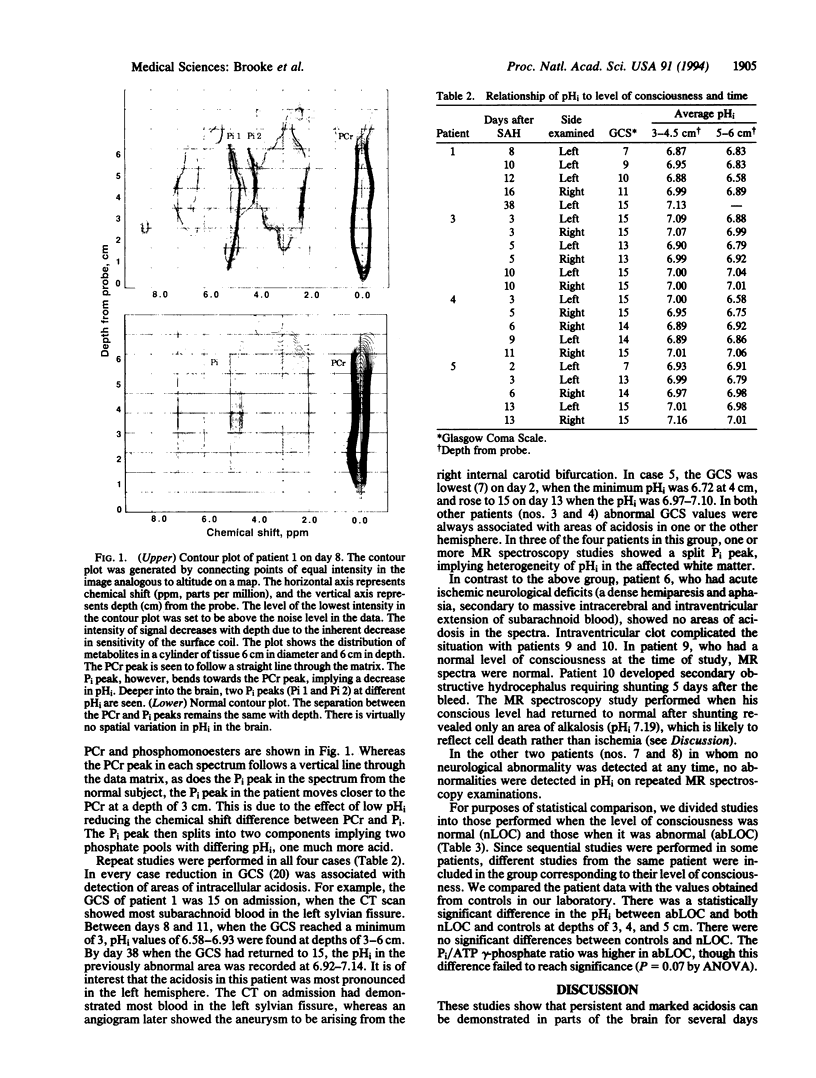
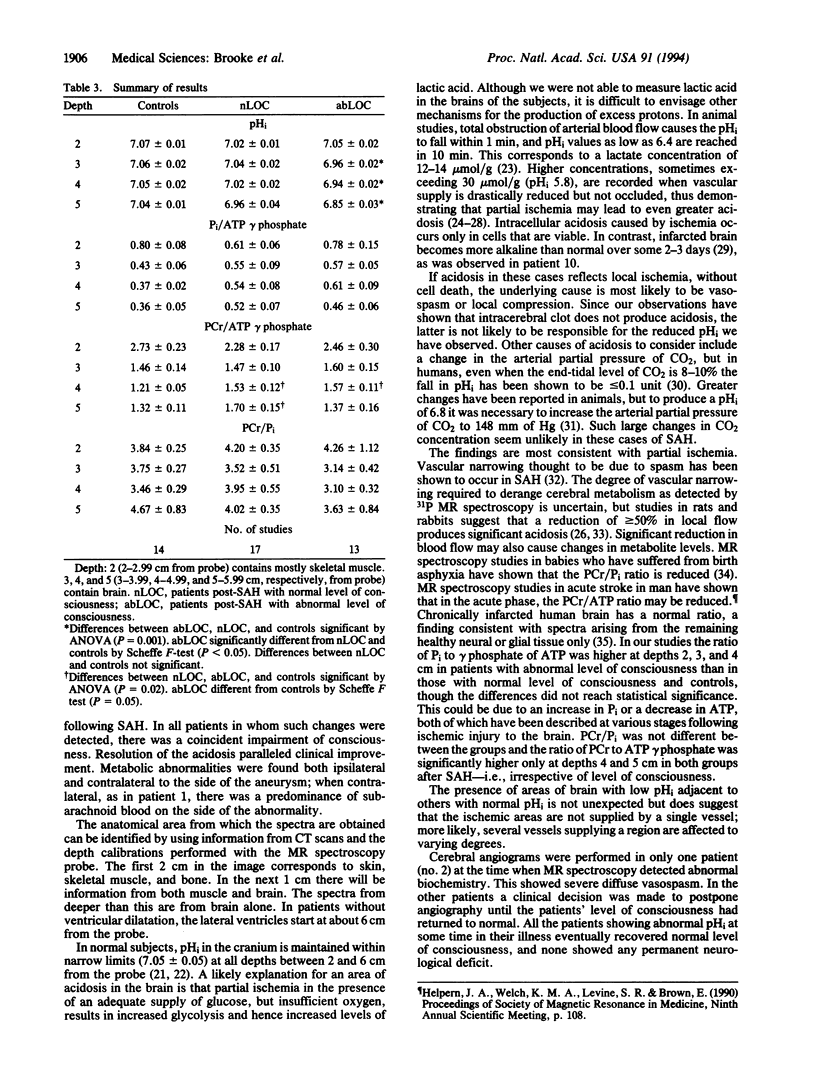

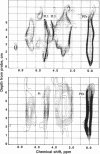
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackledge M. J., Rajagopalan B., Oberhaensli R. D., Bolas N. M., Styles P., Radda G. K. Quantitative studies of human cardiac metabolism by 31P rotating-frame NMR. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4283–4287. doi: 10.1073/pnas.84.12.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolas N. M., Rajagopalan B., Mitsumori F., Radda G. K. Metabolic changes during experimental cerebral ischemia in hyperglycemic rats, observed by 31P and 1H magnetic resonance spectroscopy. Stroke. 1988 May;19(5):608–614. doi: 10.1161/01.str.19.5.608. [DOI] [PubMed] [Google Scholar]
- Bottomley P. A., Drayer B. P., Smith L. S. Chronic adult cerebral infarction studied by phosphorus NMR spectroscopy. Radiology. 1986 Sep;160(3):763–766. doi: 10.1148/radiology.160.3.3737915. [DOI] [PubMed] [Google Scholar]
- Bryan R. N. Imaging of acute stroke. Radiology. 1990 Dec;177(3):615–616. doi: 10.1148/radiology.177.3.2243959. [DOI] [PubMed] [Google Scholar]
- Bryan R. N., Levy L. M., Whitlow W. D., Killian J. M., Preziosi T. J., Rosario J. A. Diagnosis of acute cerebral infarction: comparison of CT and MR imaging. AJNR Am J Neuroradiol. 1991 Jul-Aug;12(4):611–620. [PMC free article] [PubMed] [Google Scholar]
- Cadoux-Hudson T. A., Blackledge M. J., Rajagopalan B., Taylor D. J., Radda G. K. Human primary brain tumour metabolism in vivo: a phosphorus magnetic resonance spectroscopy study. Br J Cancer. 1989 Sep;60(3):430–436. doi: 10.1038/bjc.1989.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadoux-Hudson T. A., Rajagopalan B., Ledingham J. G., Radda G. K. Response of the human brain to a hypercapnic acid load in vivo. Clin Sci (Lond) 1990 Jul;79(1):1–3. doi: 10.1042/cs0790001. [DOI] [PubMed] [Google Scholar]
- Eklöf B., Siesjö B. K. The effect of bilateral carotid artery ligation upon acid-base parameters and substrate levels in the rat brain. Acta Physiol Scand. 1972 Dec;86(4):528–538. doi: 10.1111/j.1748-1716.1972.tb05354.x. [DOI] [PubMed] [Google Scholar]
- Eklöf B., Siesjö B. K. The effect of bilateral carotid artery ligation upon the blood flow and the energy state of the rat brain. Acta Physiol Scand. 1972 Oct;86(2):155–165. doi: 10.1111/j.1748-1716.1972.tb05322.x. [DOI] [PubMed] [Google Scholar]
- Elster A. D., Moody D. M. Early cerebral infarction: gadopentetate dimeglumine enhancement. Radiology. 1990 Dec;177(3):627–632. doi: 10.1148/radiology.177.3.2243961. [DOI] [PubMed] [Google Scholar]
- Gudmundsson G. Primary subarachnoid hemorrhage in Iceland. Stroke. 1973 Sep-Oct;4(5):764–767. doi: 10.1161/01.str.4.5.764. [DOI] [PubMed] [Google Scholar]
- Hansen B. S., Marquardsen J. Incidence of stroke in Frederiksberg, Denmark. Stroke. 1977 Nov-Dec;8(6):663–665. doi: 10.1161/01.str.8.6.663. [DOI] [PubMed] [Google Scholar]
- Hunt W. E., Hess R. M. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg. 1968 Jan;28(1):14–20. doi: 10.3171/jns.1968.28.1.0014. [DOI] [PubMed] [Google Scholar]
- Kassell N. F., Drake C. G. Timing of aneurysm surgery. Neurosurgery. 1982 Apr;10(4):514–519. doi: 10.1227/00006123-198204000-00019. [DOI] [PubMed] [Google Scholar]
- Kassell N. F., Sasaki T., Colohan A. R., Nazar G. Cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Stroke. 1985 Jul-Aug;16(4):562–572. doi: 10.1161/01.str.16.4.562. [DOI] [PubMed] [Google Scholar]
- Kassell N. F., Torner J. C., Jane J. A., Haley E. C., Jr, Adams H. P. The International Cooperative Study on the Timing of Aneurysm Surgery. Part 2: Surgical results. J Neurosurg. 1990 Jul;73(1):37–47. doi: 10.3171/jns.1990.73.1.0037. [DOI] [PubMed] [Google Scholar]
- Levine S. R., Helpern J. A., Welch K. M., Vande Linde A. M., Sawaya K. L., Brown E. E., Ramadan N. M., Deveshwar R. K., Ordidge R. J. Human focal cerebral ischemia: evaluation of brain pH and energy metabolism with P-31 NMR spectroscopy. Radiology. 1992 Nov;185(2):537–544. doi: 10.1148/radiology.185.2.1410369. [DOI] [PubMed] [Google Scholar]
- Ljunggren B., Schutz H., Siesjö B. K. Changes in energy state and acid-base parameters of the rat brain during complete compression ischemia. Brain Res. 1974 Jun 20;73(2):277–289. doi: 10.1016/0006-8993(74)91049-x. [DOI] [PubMed] [Google Scholar]
- Marshall L. F., Welsh F., Durity F., Lounsbury R., Graham D. I., Langfitt T. W. Experimental cerebral oligemia and ischemia produced by intracranial hypertension. Part 3: Brain energy metabolism. J Neurosurg. 1975 Sep;43(3):323–328. doi: 10.3171/jns.1975.43.3.0323. [DOI] [PubMed] [Google Scholar]
- Moorcraft J., Bolas N. M., Ives N. K., Sutton P., Blackledge M. J., Rajagopalan B., Hope P. L., Radda G. K. Spatially localized magnetic resonance spectroscopy of the brains of normal and asphyxiated newborns. Pediatrics. 1991 Mar;87(3):273–282. [PubMed] [Google Scholar]
- Nordström C. H., Siesjö B. K. Effects of phenobarbital in cerebral ischemia. Part I: cerebral energy metabolism during pronounced incomplete ischemia. Stroke. 1978 Jul-Aug;9(4):327–335. doi: 10.1161/01.str.9.4.327. [DOI] [PubMed] [Google Scholar]
- Oberhaensli R., Rajagopalan B., Galloway G. J., Taylor D. J., Radda G. K. Study of human liver disease with P-31 magnetic resonance spectroscopy. Gut. 1990 Apr;31(4):463–467. doi: 10.1136/gut.31.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips L. H., 2nd, Whisnant J. P., O'Fallon W. M., Sundt T. M., Jr The unchanging pattern of subarachnoid hemorrhage in a community. Neurology. 1980 Oct;30(10):1034–1040. doi: 10.1212/wnl.30.10.1034. [DOI] [PubMed] [Google Scholar]
- Pickard J. D., Murray G. D., Illingworth R., Shaw M. D., Teasdale G. M., Foy P. M., Humphrey P. R., Lang D. A., Nelson R., Richards P. Effect of oral nimodipine on cerebral infarction and outcome after subarachnoid haemorrhage: British aneurysm nimodipine trial. BMJ. 1989 Mar 11;298(6674):636–642. doi: 10.1136/bmj.298.6674.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radda G. K. The use of NMR spectroscopy for the understanding of disease. Science. 1986 Aug 8;233(4764):640–645. doi: 10.1126/science.3726553. [DOI] [PubMed] [Google Scholar]
- Rehncrona S., Rosén I., Siesjö B. K. Brain lactic acidosis and ischemic cell damage: 1. Biochemistry and neurophysiology. J Cereb Blood Flow Metab. 1981;1(3):297–311. doi: 10.1038/jcbfm.1981.34. [DOI] [PubMed] [Google Scholar]
- Siesjö B. K., Folbergrová J., MacMillan V. The effect of hypercapnia upon intracellular pH in the brain, evaluated by the bicarbonate-carbonic acid method and from the creatine phosphokinase equilibrium. J Neurochem. 1972 Nov;19(11):2483–2495. doi: 10.1111/j.1471-4159.1972.tb01308.x. [DOI] [PubMed] [Google Scholar]
- Smith M. L., von Hanwehr R., Siesjö B. K. Changes in extra- and intracellular pH in the brain during and following ischemia in hyperglycemic and in moderately hypoglycemic rats. J Cereb Blood Flow Metab. 1986 Oct;6(5):574–583. doi: 10.1038/jcbfm.1986.104. [DOI] [PubMed] [Google Scholar]
- Taylor D. J., Bore P. J., Styles P., Gadian D. G., Radda G. K. Bioenergetics of intact human muscle. A 31P nuclear magnetic resonance study. Mol Biol Med. 1983 Jul;1(1):77–94. [PubMed] [Google Scholar]
- Teasdale G., Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974 Jul 13;2(7872):81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]