Abstract
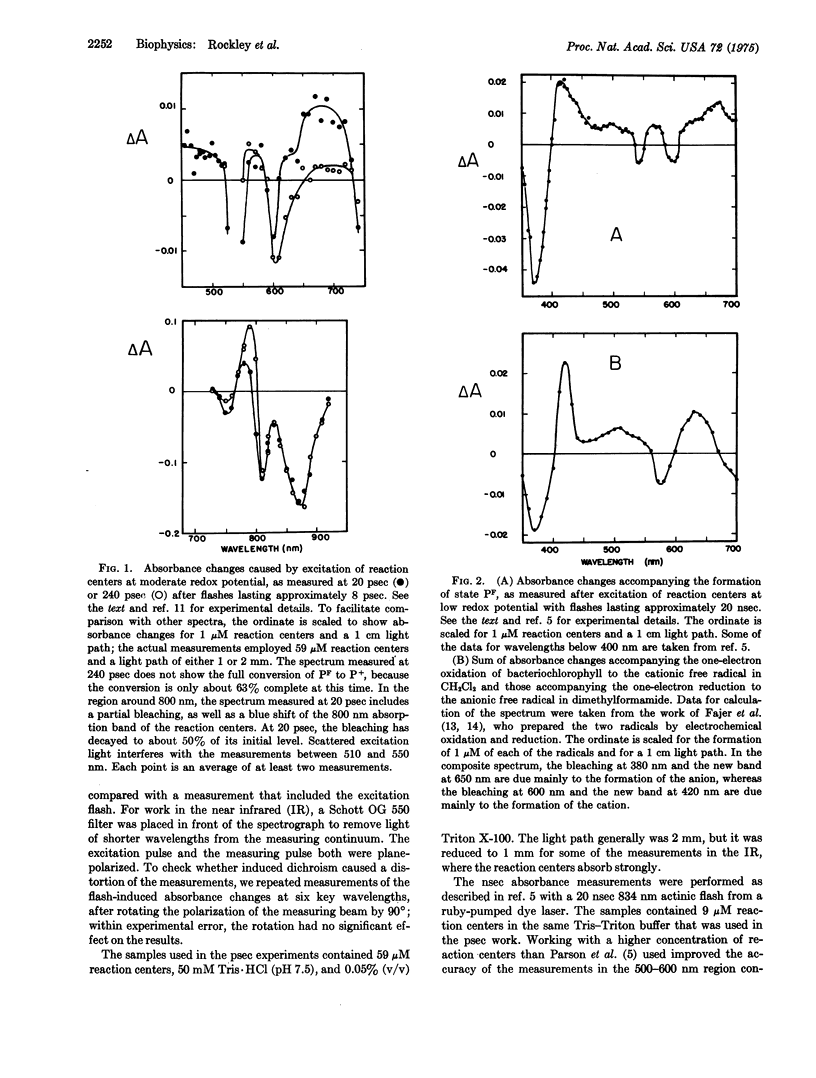
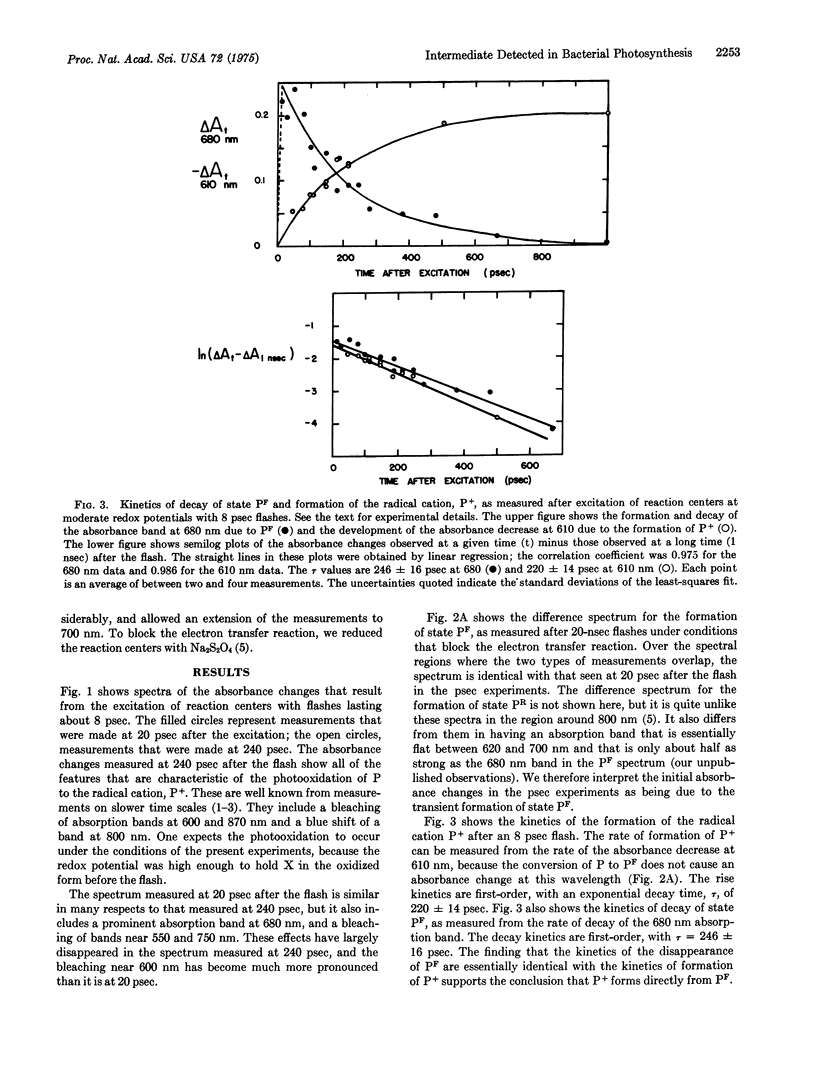
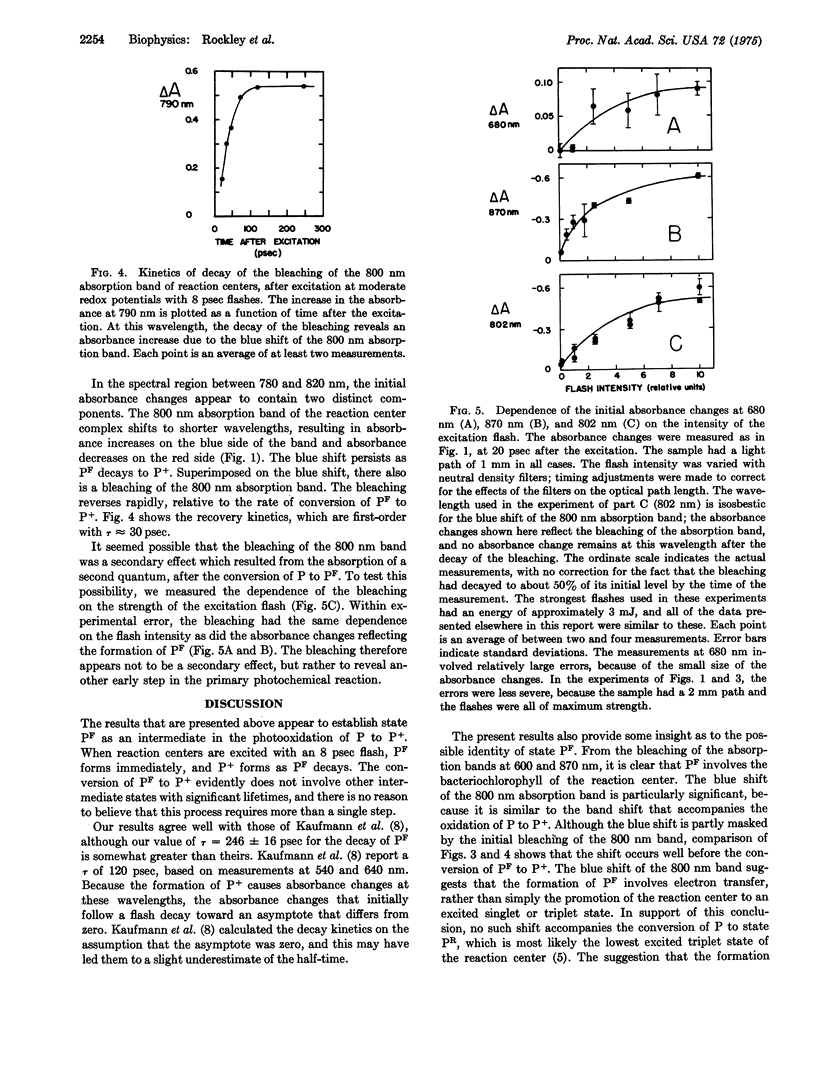
Preparations of photosynthetic reaction centers from Rhodopseudomonas sphaeroides were excited with flashes lasting approximately 8 psec. Immediately after the excitation, there appeared a transient state which was characterized by new absorption bands near 500 and 680 nm, by a bleaching of bands near 540, 600, 760, and 870 nm, and by a blue shift of a band near 800 nm. The transient state decayed with an exponential decay time,t, of 246 plus or minus 16 psec after the flash. As the transient state decayed, the radical cation of the reaction center bacteriochlorophyll complex appeared. This indicates that the transient state is an intermediate in the photooxidation of the bacteriochlorophyll. The absorpiton spectrum of the transient state shows the state to be identical with a state (P-F) which has been detected previously in reaction centers that are prevented from completing the photooxidation, because of chemical reduction of the electron acceptor. Analysis of the spectrum suggests that the formation of P-F involves electron transfer from one bacteriochlorophyll molecule to another within the reaction center, or possibly from bacteriochlorophyll to the bacteriopheophytin of the complex. The initial absorbance changes after flash excitation also include a bleaching of an absorption band at 800 nm. The bleaching decays with tau approximately equal to 30 pse. The bleaching appers not to be a secondary effect, but rather to revael another early step in the primary photochemical reaction.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clayton R. K. Primary processes in bacterial photosynthesis. Annu Rev Biophys Bioeng. 1973;2:131–156. doi: 10.1146/annurev.bb.02.060173.001023. [DOI] [PubMed] [Google Scholar]
- Dutton P. L., Leigh J. S., Wraight C. A. Direct measurement of the midpoint potential of the primary electron acceptor in Rhodopseudomonas spheroides in situ and in the isolated state: some relationships with pH and o-phenanthroline. FEBS Lett. 1973 Oct 15;36(2):169–173. doi: 10.1016/0014-5793(73)80361-8. [DOI] [PubMed] [Google Scholar]
- Fajer J., Borg D. C., Forman A., Dolphin D., Felton R. H. Anion radical of bacteriochlorophyll. J Am Chem Soc. 1973 Apr 18;95(8):2739–2741. doi: 10.1021/ja00789a085. [DOI] [PubMed] [Google Scholar]
- Fajer J., Borg D. C., Forman A., Felton R. H., Dolphin D., Vegh L. The cation radicals of free base and zinc bacteriochlorin, bacteriochlorophyll, and bacteriopheophytin. Proc Natl Acad Sci U S A. 1974 Mar;71(3):994–998. doi: 10.1073/pnas.71.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parson W. W. Bacterial photosynthesis. Annu Rev Microbiol. 1974;28(0):41–59. doi: 10.1146/annurev.mi.28.100174.000353. [DOI] [PubMed] [Google Scholar]
- Parson W. W., Cogdell R. J. The primary photochemical reaction to bacterial photosynthesis. Biochim Biophys Acta. 1975 Mar 31;416(1):105–149. doi: 10.1016/0304-4173(75)90014-2. [DOI] [PubMed] [Google Scholar]
- Reed D. W., Ke B. Spectral properties of reaction center preparations from Rhodopseudomonas spheroides. J Biol Chem. 1973 May 10;248(9):3041–3045. [PubMed] [Google Scholar]
- Sauer K., Dratz E. A., Coyne L. Circular dichroism spectra and the molecular arrangement of bacteriochlorophylls in the reaction centers of photosynthetic bacteria. Proc Natl Acad Sci U S A. 1968 Sep;61(1):17–24. doi: 10.1073/pnas.61.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slooten L. Reaction center preparations of Rhodopseudomonas spheroides: energy transfer and structure. Biochim Biophys Acta. 1972 Feb 28;256(2):452–466. doi: 10.1016/0005-2728(72)90074-6. [DOI] [PubMed] [Google Scholar]
- Uphaus R. A., Norris J. R., Katz J. J. Triplet states in photosynthesis. Biochem Biophys Res Commun. 1974 Dec 11;61(3):1057–1063. doi: 10.1016/0006-291x(74)90262-9. [DOI] [PubMed] [Google Scholar]
- Zankel K. L., Reed D. W., Clayton R. K. Fluorescence and photochemical quenching in photosynthetic reaction centers. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1243–1249. doi: 10.1073/pnas.61.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]


