Abstract
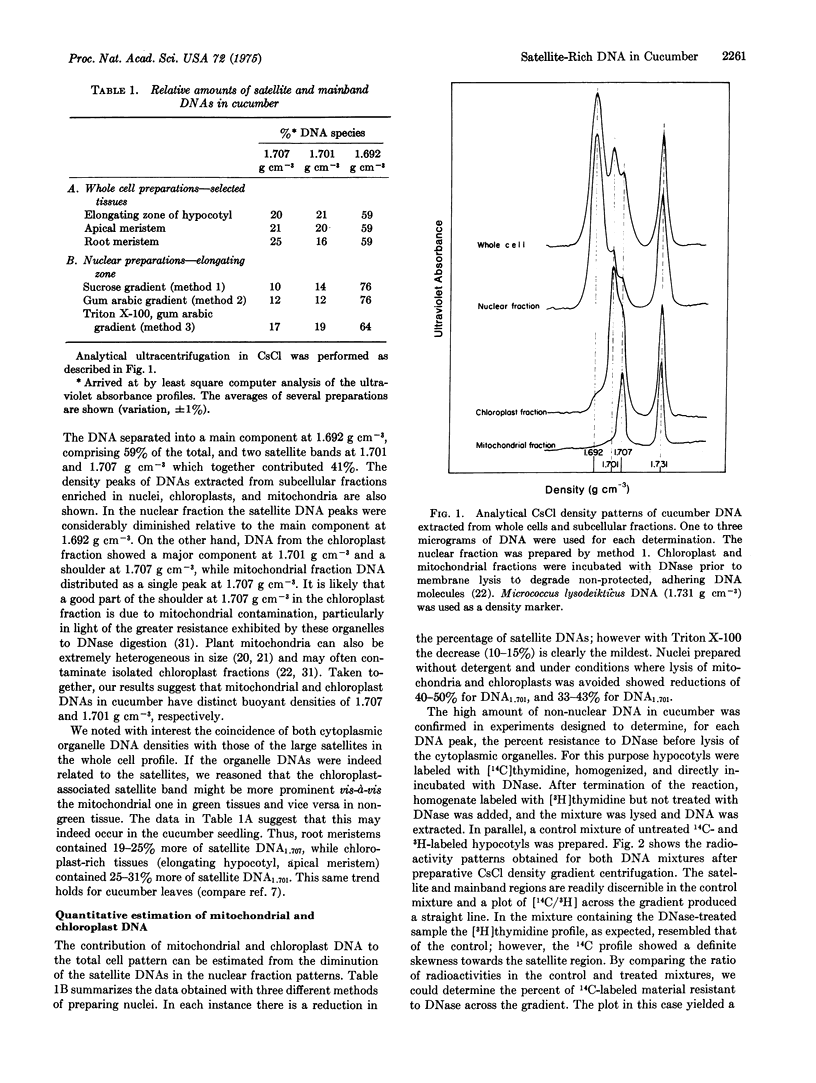
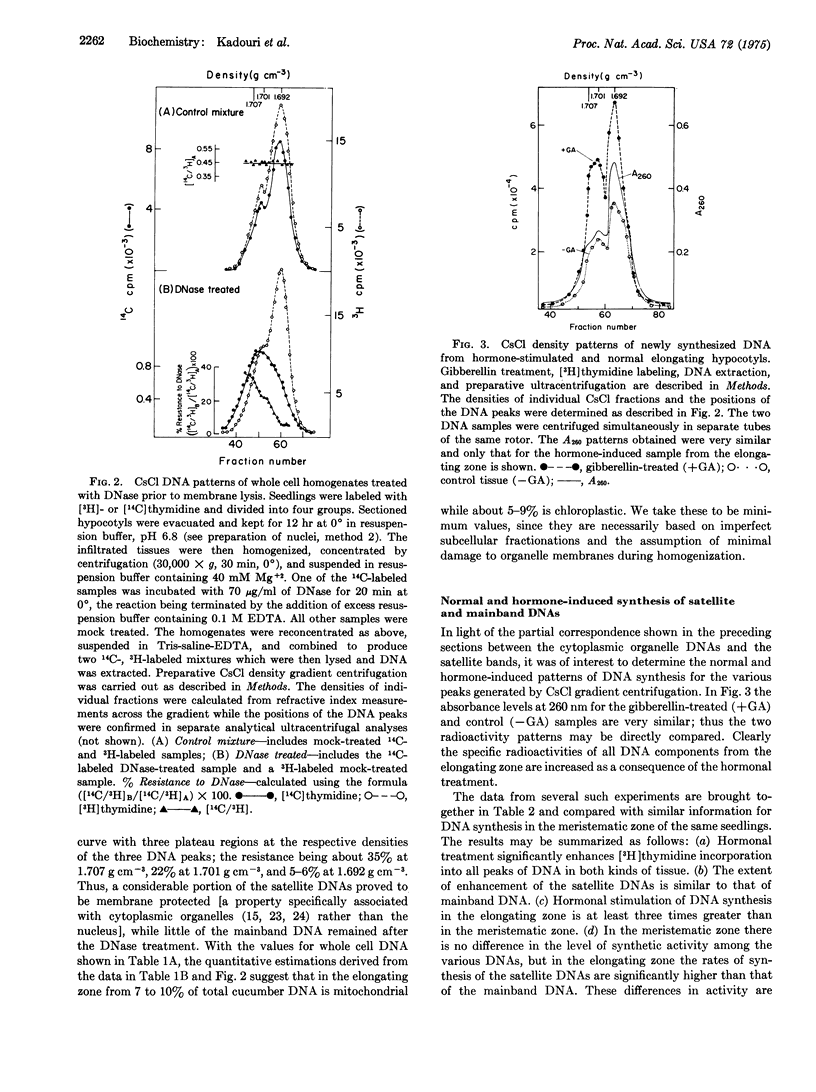
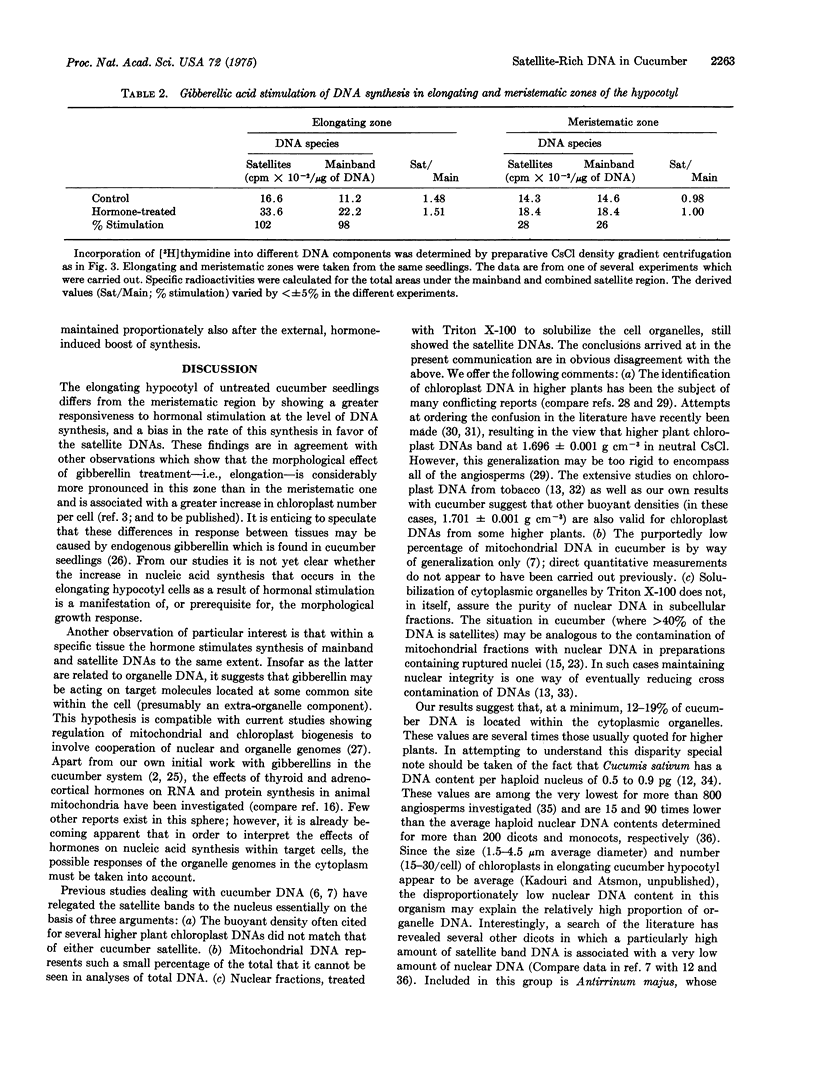
Cucumber hypocotyl DNA in neutral CsCl distributes into a mainband comprising 59% of the total, and two large satellite bands which contribute 41% to the DNA pattern. Organelle enrichment studies show that the densities of mitochondrial and chloroplast DNA coincide with those of the satellite bands. At least 12-19% of total cucumber DNA is associated with the cytoplasmic organelles. These values, which are several times larger than those usually quoted for higher plants, are correlated with an unusually low amount of DNA per haploid nucleus in cucumber. Synthesis of the satellite DNAs, as well as mainband DNA, is appreciably stimulated in vivo by application of the plant hormone, gibberellin. Endogenous and hormone-enhanced synthesis of the satellite DNAs is proportionately greater in target tissue showing a high rate of organelle synthetic activity.
Keywords: chloroplast DNA, mitochondrial DNA, gibberellin, hypocotyl elongation
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atsmon D., Lang A., Light E. N. Contents and recovery of gibberellins in monoecious and gynoecious cucumber plants. Plant Physiol. 1968 May;43(5):806–810. doi: 10.1104/pp.43.5.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendich A. J., Anderson R. S. Novel properties of satellite DNA from muskmelon. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1511–1515. doi: 10.1073/pnas.71.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. D. Nuclear DNA content and minimum generation time in herbaceous plants. Proc R Soc Lond B Biol Sci. 1972 Jun 6;181(1063):109–135. doi: 10.1098/rspb.1972.0042. [DOI] [PubMed] [Google Scholar]
- Calvayrac R., Butow R. A., Lefort-Tran M. Cyclic replication of DNA and changes in mitochondrial morphology during the cell cycle of Euglena gracilis (Z). Exp Cell Res. 1972;71(2):422–432. doi: 10.1016/0014-4827(72)90312-6. [DOI] [PubMed] [Google Scholar]
- Degani Y., Atsmon D. Enhancement of non-nuclear DNA synthesis associated with hormone-induced elongation in the cucumber hypocotyl. Exp Cell Res. 1970 Jul;61(1):226–229. doi: 10.1016/0014-4827(70)90283-1. [DOI] [PubMed] [Google Scholar]
- Degani Y., Atsmon D., Halevy A. H. DNA synthesis and hormone-induced elongation in the cucumber hypocotyl. Nature. 1970 Nov 7;228(5271):554–555. doi: 10.1038/228554a0. [DOI] [PubMed] [Google Scholar]
- Edelman M., Epstein H. T., Schiff J. A. Isolation and characterization of DNA from the mitochondrial fraction of Euglena. J Mol Biol. 1966 Jun;17(2):463–469. doi: 10.1016/s0022-2836(66)80156-0. [DOI] [PubMed] [Google Scholar]
- Gadaleta M. N., Barletta A., Caldarazzo M., De Leo T., Saccone C. Triiodothyronine action on RNA synthesis in rat-liver mitochondria. Eur J Biochem. 1972 Oct;30(2):376–381. doi: 10.1111/j.1432-1033.1972.tb02108.x. [DOI] [PubMed] [Google Scholar]
- Green B., Heilporn V., Limbosch S., Boloukhere M., Brachet J. The cytoplasmic DNA's of Acetabularia mediterranea. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1351–1358. doi: 10.1073/pnas.58.4.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann R. G., Bohnert H. J., Kowallik K. V., Schmitt J. M. Size, conformation and purity of chloroplast DNA of some higher plants. Biochim Biophys Acta. 1975 Jan 20;378(2):305–317. doi: 10.1016/0005-2787(75)90118-5. [DOI] [PubMed] [Google Scholar]
- Ingle J., Pearson G. G., Sinclair J. Species distribution and properties of nuclear satellite DNA in higher plants. Nat New Biol. 1973 Apr 18;242(120):193–197. doi: 10.1038/newbio242193a0. [DOI] [PubMed] [Google Scholar]
- Mache R., Waygood E. R. Characterization of DNA in wheat chloroplasts isolated by a new "laceration technique". FEBS Lett. 1969 Apr;3(2):89–92. doi: 10.1016/0014-5793(69)80104-3. [DOI] [PubMed] [Google Scholar]
- Matsuda K., Siegel A. Hybridization of plant ribosomal RNA to DNA: the isolation of a DNA component rich in ribosomal RNA cistrons. Proc Natl Acad Sci U S A. 1967 Aug;58(2):673–680. doi: 10.1073/pnas.58.2.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda K., Siegel A., Lightfoot D. Variability in Complementarity for Chloroplastic and Cytoplasmic Ribosomal Ribonucleic Acid among Plant Nuclear Deoxyribonucleic Acids. Plant Physiol. 1970 Jul;46(1):6–12. doi: 10.1104/pp.46.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow A. H., Price H. J., Underbrink A. G. A survey of DNA content per cell and per chromosome of prokaryotic and eukaryotic organisms: some evolutionary considerations. Brookhaven Symp Biol. 1972;23:451–494. [PubMed] [Google Scholar]
- Tautvydas K. J. Mass isolation of pea nuclei. Plant Physiol. 1971 Apr;47(4):499–503. doi: 10.1104/pp.47.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari K. K., Wildman S. G. Chloroplast DNA from tobacco leaves. Science. 1966 Sep 9;153(3741):1269–1271. doi: 10.1126/science.153.3741.1269. [DOI] [PubMed] [Google Scholar]
- Tewari K. K., Wildman S. G. Information content in the chloroplast DNA. Symp Soc Exp Biol. 1970;24:147–179. [PubMed] [Google Scholar]
- Thornburg W., Siegel A. Characteristics of the rapidly reassociating deoxyribonucleic acid of Cucurbita pepo L. and the sequences complementary to ribosomal and transfer ribonucleic acids. Biochemistry. 1973 Jul 3;12(14):2759–2765. doi: 10.1021/bi00738a032. [DOI] [PubMed] [Google Scholar]
- Walker P. M. "Repetitive" DNA in higher organisms. Prog Biophys Mol Biol. 1971;23:145–190. doi: 10.1016/0079-6107(71)90019-8. [DOI] [PubMed] [Google Scholar]
- Wells R., Birnstiel M. Kinetic complexity of chloroplastal deoxyribonucleic acid and mitochondrial deoxyribonucleic acid from higher plants. Biochem J. 1969 May;112(5):777–786. doi: 10.1042/bj1120777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells R., Ingle J. The constancy of the buoyant density of chloroplast and mitochondrial deoxyribonucleic acids in a range of higher plants. Plant Physiol. 1970 Jul;46(1):178–179. doi: 10.1104/pp.46.1.178. [DOI] [PMC free article] [PubMed] [Google Scholar]


