Abstract
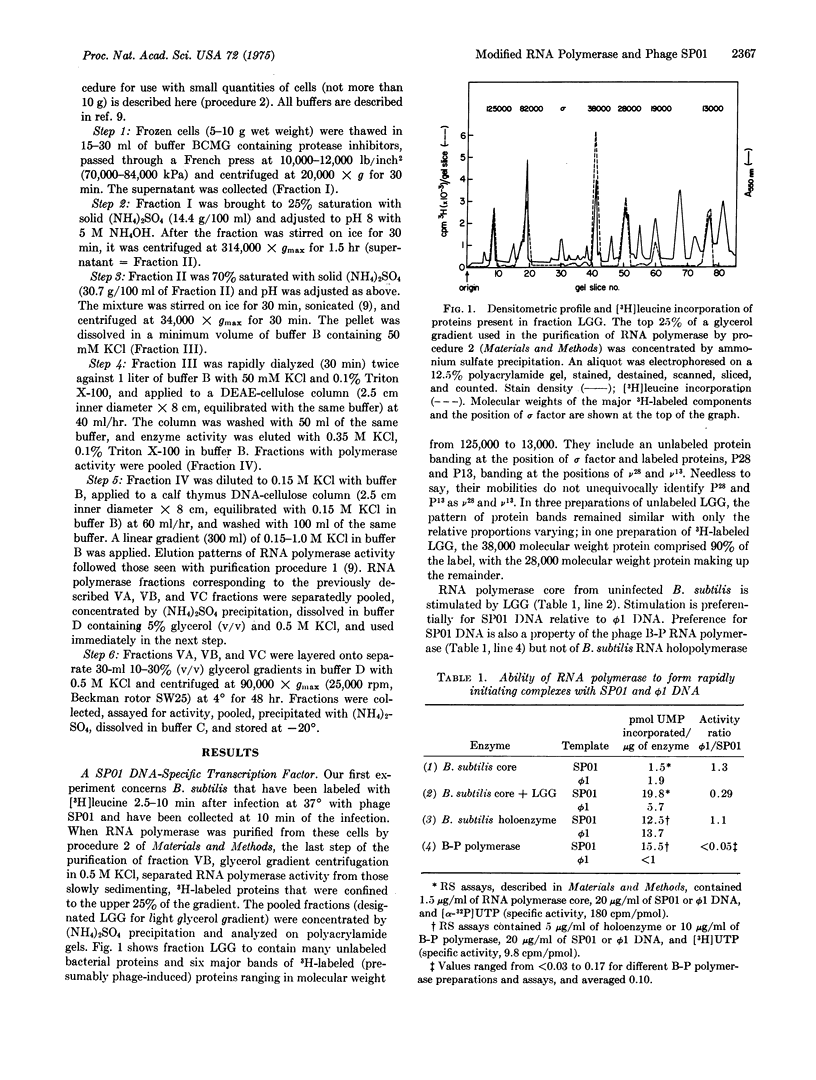
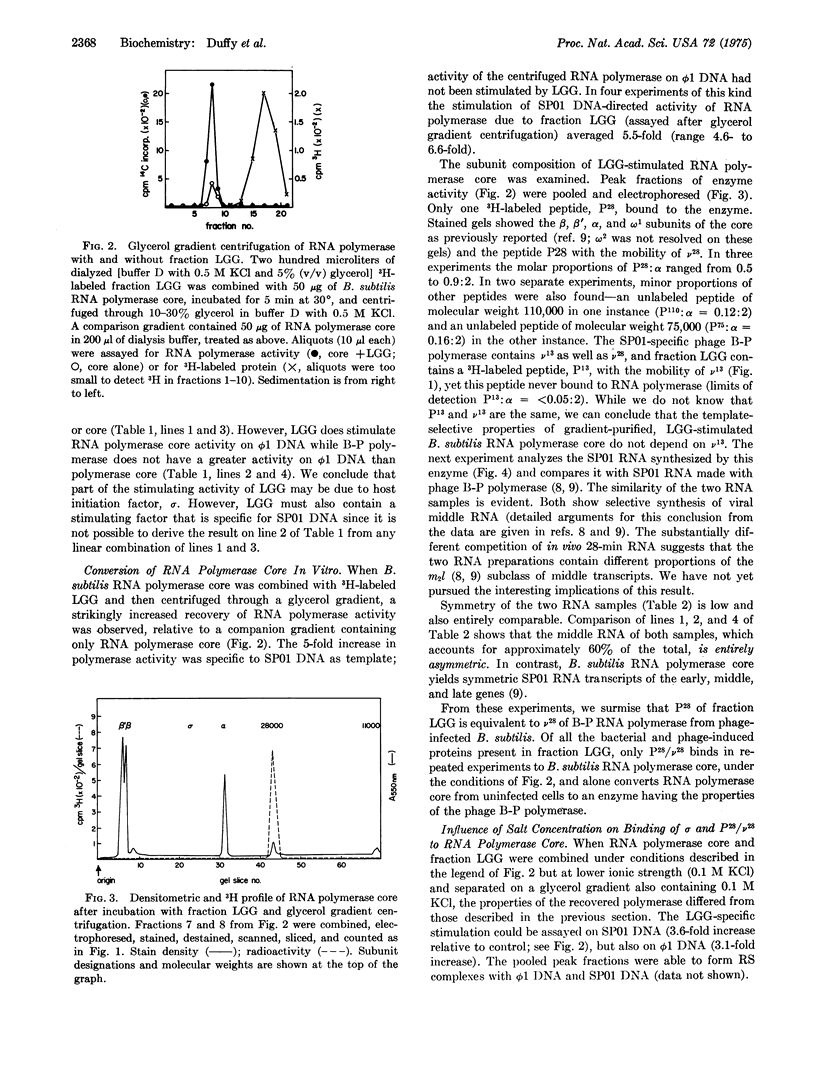
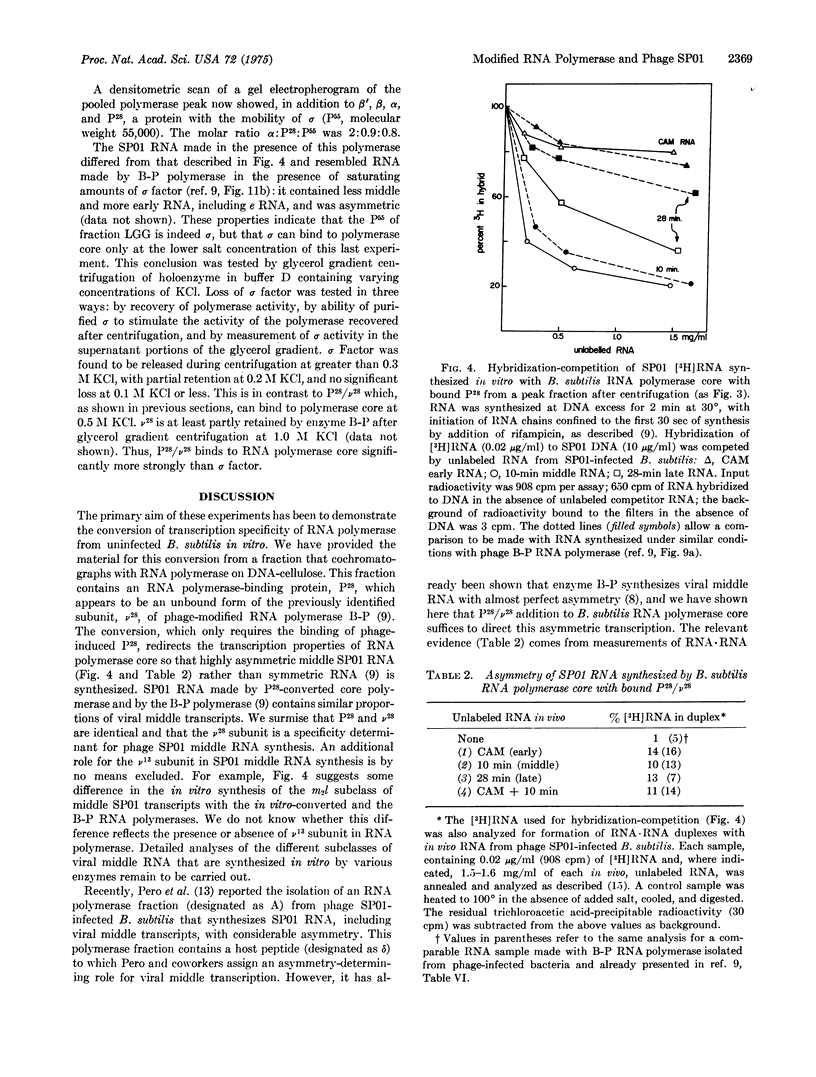
A protein fraction from B. subtilis infected with phage SP01 (fraction LGG) stimulates the activity of RNA polymerase (EC 2.7.7.6; nucleosidetriphosphate:RNA nucleotidyltransferase) core from uninfected bacteria. Fraction LGG contains a protein (P-28, molecular weight 28,000) that is labeled after phage infection and binds tightly to RNA polymerase core at a relatively high ionic strength. B. subtilis RNA polymerase core with bound P-28 has the transcription specificity of the previously purified, phage-modified B-P RNA polymerase; the latter contains two subunits, v-28 and v-13 (molecular weights 28,000 and 13,000, respectively) that are synthesized after phage infection. Both enzymes transcribe SP01 DNA preferentially and direct the asymmetric synthesis of viral middle RNA. P-28, like v-28, binds more tightly to B. subtilis RNA polymerase core than the B. subtilis initiation factor, sigma, at higher ionic strength. We propose that P-28 and v-28 are the same protein. P-28 and, by implication, v-28 suffice to endow the bacterial RNA polymerase core with a novel transcription specificity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolle A., Epstein R. H., Salser W., Geiduschek E. P. Transcription during bacteriophage T4 development: synthesis and relative stability of early and late RNA. J Mol Biol. 1968 Feb 14;31(3):325–348. doi: 10.1016/0022-2836(68)90413-0. [DOI] [PubMed] [Google Scholar]
- Chamberlin M. J. The selectivity of transcription. Annu Rev Biochem. 1974;43(0):721–775. doi: 10.1146/annurev.bi.43.070174.003445. [DOI] [PubMed] [Google Scholar]
- Dharmgrongartama B., Mahadik S. P., Srinivasan P. R. Modification of RNA polymerase after T3 phage infection of Escherichia coli B. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2845–2849. doi: 10.1073/pnas.70.10.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy J. J., Geiduschek E. P. Transcription specificity of an RNA polymerase fraction from bacteriophage SP01-infected B. subtilis. FEBS Lett. 1973 Aug 15;34(2):172–174. doi: 10.1016/0014-5793(73)80786-0. [DOI] [PubMed] [Google Scholar]
- Fox T. D., Pero J. New phage-SPO1-induced polypeptides associated with Bacillus subtilis RNA polymerase. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2761–2765. doi: 10.1073/pnas.71.7.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEIDUSCHEK E. P., MOOHR J. W., WEISS S. B. The secondary structure of complementary RNA. Proc Natl Acad Sci U S A. 1962 Jun 15;48:1078–1086. doi: 10.1073/pnas.48.6.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz H. R. Control by bacteriophage T4 of two sequential phosphorylations of the alpha subunit of Escherichia coli RNA polymerase. J Mol Biol. 1974 Dec 25;90(4):727–738. doi: 10.1016/0022-2836(74)90536-1. [DOI] [PubMed] [Google Scholar]
- Horvitz H. R. Polypeptide bound to the host RNA polymerase is specified by T4 control gene 33. Nat New Biol. 1973 Aug 1;244(135):137–140. doi: 10.1038/newbio244137a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ratner D. Letter to the editor: Bacteriophage T4 transcriptional control gene 55 codes for a protein bound to Escherichia coli RNA polymerase. J Mol Biol. 1974 Nov 15;89(4):803–807. doi: 10.1016/0022-2836(74)90054-0. [DOI] [PubMed] [Google Scholar]
- Spiegelman G. B., Whiteley H. R. Purification of ribonucleic acid polymerase from SP82-infected Bacillus subtilis. J Biol Chem. 1974 Mar 10;249(5):1476–1482. [PubMed] [Google Scholar]
- Stevens A. New small polypeptides associated with DNA-dependent RNA polymerase of Escherichia coli after infection with bacteriophage T4. Proc Natl Acad Sci U S A. 1972 Mar;69(3):603–607. doi: 10.1073/pnas.69.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]


