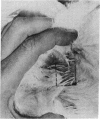
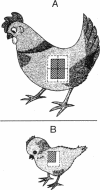
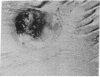
Abstract
Transfer of thymus cells from young chickens to syngeneic recipients suppresses the allograft rejection between strains differing at the major histocompatibility (B) locus. Thymus cell transfer in combination with a light whole body irradiation (360 R) prolongs significantly the mean rejection time of skin allografts and leads in a proportion of recipients to long-lasting graft survival (>200 days). Three weeks after the cell transfer, the suppression appears to be antigen specific, as judged by the normal reactivity against third-party skin grafts. From the types of thymus cell preparations that are effective in these experiments, it is inferred that the suppressor cell is a bursa-dependent lymphocyte, which is predominantly found in the young chicken thymus and which is different from B-lymphocytes, B-precursor cells, or graft-versus-host-reactive T-cells.
Keywords: suppressor cells, immunosuppression, skin graft rejection
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BILLINGHAM R. E., BRENT L., MEDAWAR P. B. Actively acquired tolerance of foreign cells. Nature. 1953 Oct 3;172(4379):603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- BILLINGHAM R. E., BRENT L., MEDAWAR P. B. Extraction of antigens causing transplantation immunity. Transplant Bull. 1958 Oct;5(4):377–381. doi: 10.1097/00006534-195810000-00034. [DOI] [PubMed] [Google Scholar]
- Droege W. Amplifying and suppressive effect of thymus cells. Nature. 1971 Dec 31;234(5331):549–551. doi: 10.1038/234549a0. [DOI] [PubMed] [Google Scholar]
- Droege W. Comparison of the suppressive effect of thymus cells and the suppression by neonatal application of antigen. Eur J Immunol. 1973 Dec;3(12):804–811. doi: 10.1002/eji.1830031213. [DOI] [PubMed] [Google Scholar]
- Droege W., Malchow D., Strominger J. L., Linna T. J. Cellular heterogeneity in the thymus: graft-versus-host activity of fractionated thymus cells in the chicken. Proc Soc Exp Biol Med. 1973 May;143(1):249–251. doi: 10.3181/00379727-143-37296. [DOI] [PubMed] [Google Scholar]
- Droege W., Zucker R., Hannig K. Developmental changes in the cellular composition of the chicken thymus. Cell Immunol. 1974 May;12(2):186–193. doi: 10.1016/0008-8749(74)90071-9. [DOI] [PubMed] [Google Scholar]
- Feldman M. Induction fo B cell tolerance by antigen specific T cell factor. Nat New Biol. 1973 Mar 21;242(116):82–84. [PubMed] [Google Scholar]
- Gershon R. K. T cell control of antibody production. Contemp Top Immunobiol. 1974;3:1–40. doi: 10.1007/978-1-4684-3045-5_1. [DOI] [PubMed] [Google Scholar]
- HASEK M. Tolerance phenomena in birds. Proc R Soc Lond B Biol Sci. 1956 Nov 13;146(922):67–77. doi: 10.1098/rspb.1956.0072. [DOI] [PubMed] [Google Scholar]
- MEDAWAR P. B. The use of antigenic tissue extracts to weaken the immunological reaction against skin homografts in mice. Transplantation. 1963 Jan;1:21–38. doi: 10.1097/00007890-196301010-00004. [DOI] [PubMed] [Google Scholar]
- Playfair J. H. The role of antibody in T-cell responses. Clin Exp Immunol. 1974 May;17(1):1–18. [PMC free article] [PubMed] [Google Scholar]
- Toivanen P., Toivanen A., Good R. A. Ontogeny of bursal function in chicken. 3. Immunocompletent cell for humoral immunity. J Exp Med. 1972 Oct 1;136(4):816–831. doi: 10.1084/jem.136.4.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toivanen P., Toivanen A., Taamminen P. Bursal and postbursal cells in chicken. Occurrence of postbursal cells in bone marrow, thymus and spleen. Eur J Immunol. 1974 Jun;4(6):405–410. doi: 10.1002/eji.1830040604. [DOI] [PubMed] [Google Scholar]
- Zucker R., Jauker U., Droege W. Cellular composition of the chicken thymus; effects of neonatal bursectomy and hydrocortisone treatment. Eur J Immunol. 1973 Dec;3(12):812–818. doi: 10.1002/eji.1830031214. [DOI] [PubMed] [Google Scholar]