Abstract
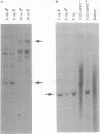
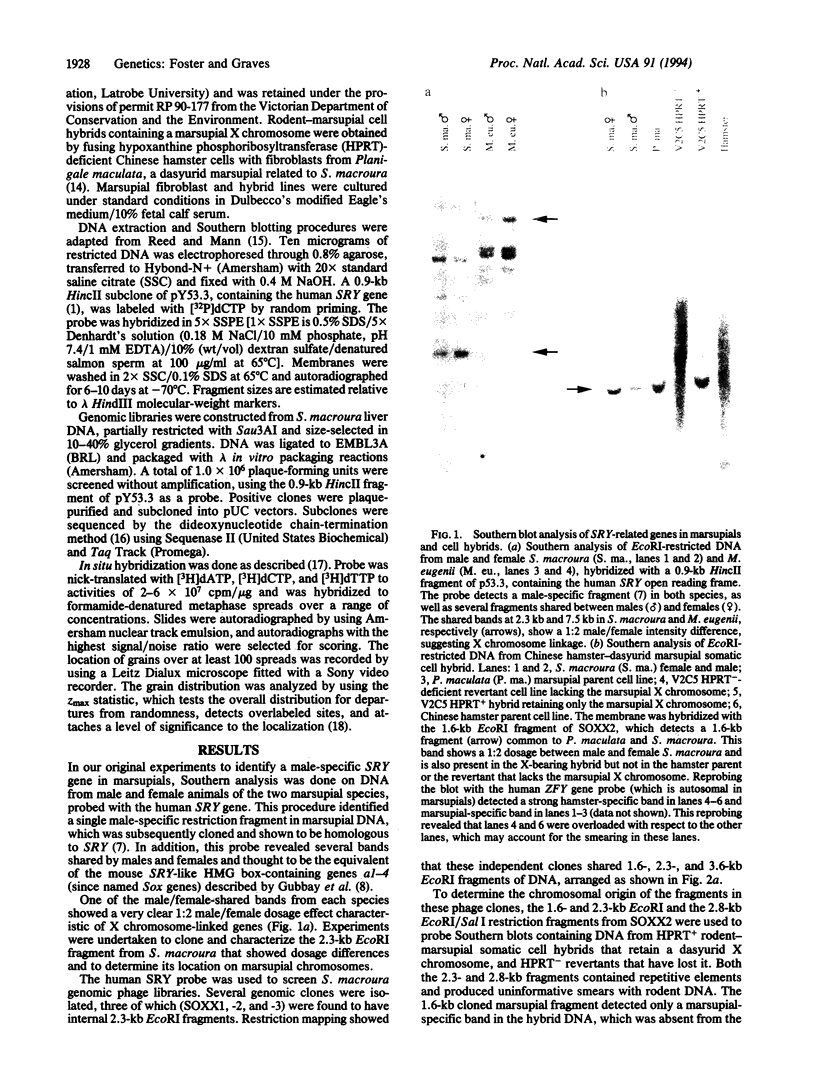
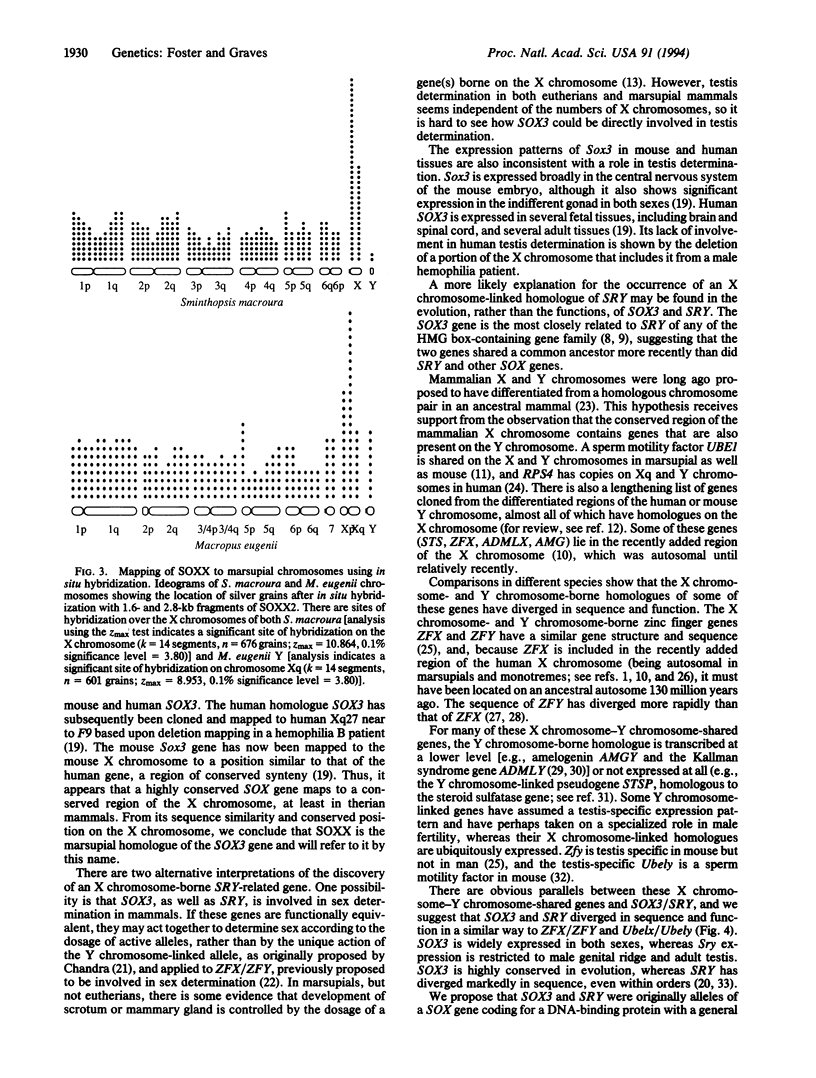
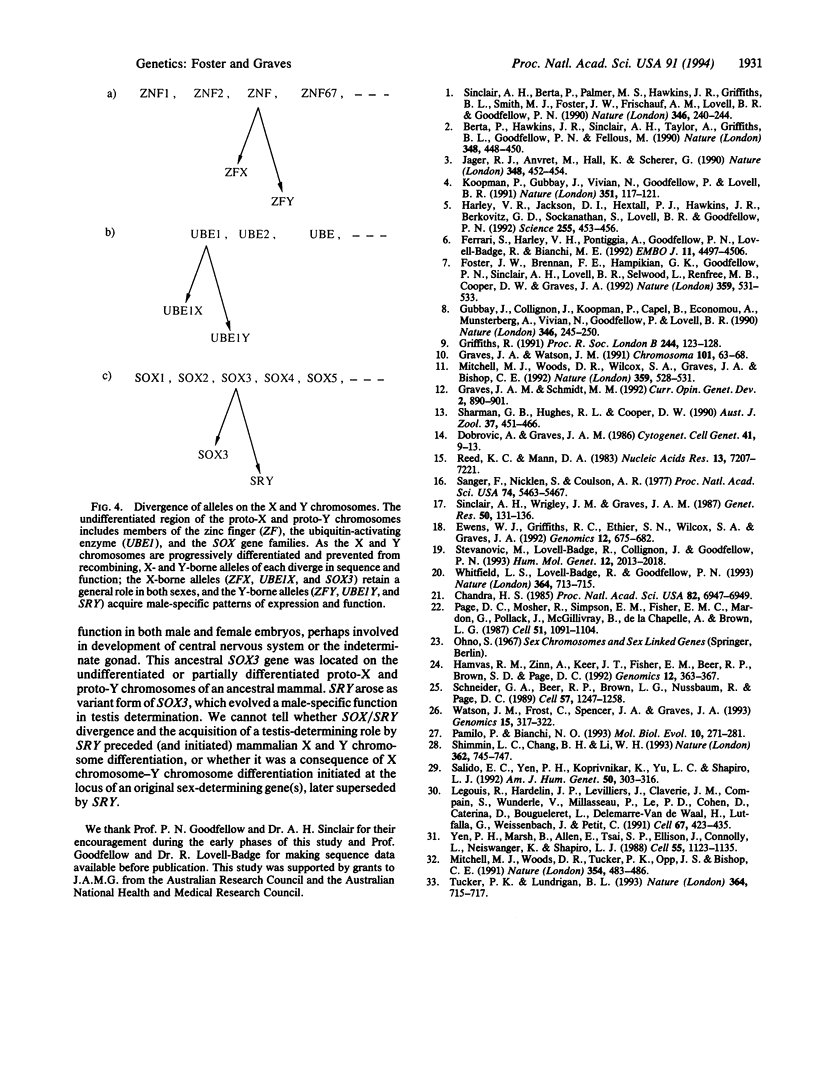
The SRY gene on the human, mouse, and marsupial Y chromosomes is the testis-determining gene that initiates male development in mammals. The SRY protein has a DNA-binding domain (high mobility group or HMG box) similar to those found in the high-mobility-group proteins. SRY is specific for the Y chromosome, but many autosomal genes have been identified that possess a similar HMG box region; those with the most closely SRY-related box regions form a gene family now referred to as SOX genes. We have identified a sequence on the marsupial X chromosome that shares homology with SRY. Sequence comparisons show near-identity with the mouse and human SOX3 gene (formerly called a3), the SOX gene which is the most closely related to SRY. We suggest here that the highly conserved X chromosome-linked SOX3 represents the ancestral SOX gene from which the sex-determining gene SRY was derived. In this model SOX3/SRY divergence and the acquisition of a testis-determining role by SRY might have preceded (and initiated) sex chromosome differentiation or, alternatively, might have been a consequence of X chromosome-Y chromosome differentiation initiated at the locus of an original sex-determining gene(s), later superseded by SRY.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berta P., Hawkins J. R., Sinclair A. H., Taylor A., Griffiths B. L., Goodfellow P. N., Fellous M. Genetic evidence equating SRY and the testis-determining factor. Nature. 1990 Nov 29;348(6300):448–450. doi: 10.1038/348448A0. [DOI] [PubMed] [Google Scholar]
- Chandra H. S. Is human X chromosome inactivation a sex-determining device? Proc Natl Acad Sci U S A. 1985 Oct;82(20):6947–6949. doi: 10.1073/pnas.82.20.6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewens W. J., Griffiths R. C., Ethier S. N., Wilcox S. A., Graves J. A. Statistical analysis of in situ hybridization data: derivation and use of the zmax test. Genomics. 1992 Apr;12(4):675–682. doi: 10.1016/0888-7543(92)90293-2. [DOI] [PubMed] [Google Scholar]
- Ferrari S., Harley V. R., Pontiggia A., Goodfellow P. N., Lovell-Badge R., Bianchi M. E. SRY, like HMG1, recognizes sharp angles in DNA. EMBO J. 1992 Dec;11(12):4497–4506. doi: 10.1002/j.1460-2075.1992.tb05551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. W., Brennan F. E., Hampikian G. K., Goodfellow P. N., Sinclair A. H., Lovell-Badge R., Selwood L., Renfree M. B., Cooper D. W., Graves J. A. Evolution of sex determination and the Y chromosome: SRY-related sequences in marsupials. Nature. 1992 Oct 8;359(6395):531–533. doi: 10.1038/359531a0. [DOI] [PubMed] [Google Scholar]
- Graves J. A., Schmidt M. M. Mammalian sex chromosomes: design or accident? Curr Opin Genet Dev. 1992 Dec;2(6):890–901. doi: 10.1016/s0959-437x(05)80112-1. [DOI] [PubMed] [Google Scholar]
- Graves J. A., Watson J. M. Mammalian sex chromosomes: evolution of organization and function. Chromosoma. 1991 Nov;101(2):63–68. doi: 10.1007/BF00357055. [DOI] [PubMed] [Google Scholar]
- Griffiths R. The isolation of conserved DNA sequences related to the human sex-determining region Y gene from the lesser black-backed gull (Larus fuscus). Proc Biol Sci. 1991 May 22;244(1310):123–128. doi: 10.1098/rspb.1991.0060. [DOI] [PubMed] [Google Scholar]
- Gubbay J., Collignon J., Koopman P., Capel B., Economou A., Münsterberg A., Vivian N., Goodfellow P., Lovell-Badge R. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature. 1990 Jul 19;346(6281):245–250. doi: 10.1038/346245a0. [DOI] [PubMed] [Google Scholar]
- Hamvas R. M., Zinn A., Keer J. T., Fisher E. M., Beer-Romero P., Brown S. D., Page D. C. Rps4 maps near the inactivation center on the mouse X chromosome. Genomics. 1992 Feb;12(2):363–367. doi: 10.1016/0888-7543(92)90386-7. [DOI] [PubMed] [Google Scholar]
- Harley V. R., Jackson D. I., Hextall P. J., Hawkins J. R., Berkovitz G. D., Sockanathan S., Lovell-Badge R., Goodfellow P. N. DNA binding activity of recombinant SRY from normal males and XY females. Science. 1992 Jan 24;255(5043):453–456. doi: 10.1126/science.1734522. [DOI] [PubMed] [Google Scholar]
- Jäger R. J., Anvret M., Hall K., Scherer G. A human XY female with a frame shift mutation in the candidate testis-determining gene SRY. Nature. 1990 Nov 29;348(6300):452–454. doi: 10.1038/348452a0. [DOI] [PubMed] [Google Scholar]
- Koopman P., Gubbay J., Vivian N., Goodfellow P., Lovell-Badge R. Male development of chromosomally female mice transgenic for Sry. Nature. 1991 May 9;351(6322):117–121. doi: 10.1038/351117a0. [DOI] [PubMed] [Google Scholar]
- Legouis R., Hardelin J. P., Levilliers J., Claverie J. M., Compain S., Wunderle V., Millasseau P., Le Paslier D., Cohen D., Caterina D. The candidate gene for the X-linked Kallmann syndrome encodes a protein related to adhesion molecules. Cell. 1991 Oct 18;67(2):423–435. doi: 10.1016/0092-8674(91)90193-3. [DOI] [PubMed] [Google Scholar]
- Mitchell M. J., Woods D. R., Tucker P. K., Opp J. S., Bishop C. E. Homology of a candidate spermatogenic gene from the mouse Y chromosome to the ubiquitin-activating enzyme E1. Nature. 1991 Dec 12;354(6353):483–486. doi: 10.1038/354483a0. [DOI] [PubMed] [Google Scholar]
- Mitchell M. J., Woods D. R., Wilcox S. A., Graves J. A., Bishop C. E. Marsupial Y chromosome encodes a homologue of the mouse Y-linked candidate spermatogenesis gene Ube1y. Nature. 1992 Oct 8;359(6395):528–531. doi: 10.1038/359528a0. [DOI] [PubMed] [Google Scholar]
- Page D. C., Mosher R., Simpson E. M., Fisher E. M., Mardon G., Pollack J., McGillivray B., de la Chapelle A., Brown L. G. The sex-determining region of the human Y chromosome encodes a finger protein. Cell. 1987 Dec 24;51(6):1091–1104. doi: 10.1016/0092-8674(87)90595-2. [DOI] [PubMed] [Google Scholar]
- Pamilo P., Bianchi N. O. Evolution of the Zfx and Zfy genes: rates and interdependence between the genes. Mol Biol Evol. 1993 Mar;10(2):271–281. doi: 10.1093/oxfordjournals.molbev.a040003. [DOI] [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salido E. C., Yen P. H., Koprivnikar K., Yu L. C., Shapiro L. J. The human enamel protein gene amelogenin is expressed from both the X and the Y chromosomes. Am J Hum Genet. 1992 Feb;50(2):303–316. [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Gädicke A., Beer-Romero P., Brown L. G., Nussbaum R., Page D. C. ZFX has a gene structure similar to ZFY, the putative human sex determinant, and escapes X inactivation. Cell. 1989 Jun 30;57(7):1247–1258. doi: 10.1016/0092-8674(89)90061-5. [DOI] [PubMed] [Google Scholar]
- Shimmin L. C., Chang B. H., Li W. H. Male-driven evolution of DNA sequences. Nature. 1993 Apr 22;362(6422):745–747. doi: 10.1038/362745a0. [DOI] [PubMed] [Google Scholar]
- Sinclair A. H., Berta P., Palmer M. S., Hawkins J. R., Griffiths B. L., Smith M. J., Foster J. W., Frischauf A. M., Lovell-Badge R., Goodfellow P. N. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature. 1990 Jul 19;346(6281):240–244. doi: 10.1038/346240a0. [DOI] [PubMed] [Google Scholar]
- Sinclair A. H., Wrigley J. M., Marshall Graves J. A. Autosomal assignment of OTC in marsupials and monotremes: implications for the evolution of sex chromosomes. Genet Res. 1987 Oct;50(2):131–136. doi: 10.1017/s0016672300023533. [DOI] [PubMed] [Google Scholar]
- Stevanović M., Lovell-Badge R., Collignon J., Goodfellow P. N. SOX3 is an X-linked gene related to SRY. Hum Mol Genet. 1993 Dec;2(12):2013–2018. doi: 10.1093/hmg/2.12.2013. [DOI] [PubMed] [Google Scholar]
- Tucker P. K., Lundrigan B. L. Rapid evolution of the sex determining locus in Old World mice and rats. Nature. 1993 Aug 19;364(6439):715–717. doi: 10.1038/364715a0. [DOI] [PubMed] [Google Scholar]
- Watson J. M., Frost C., Spencer J. A., Graves J. A. Sequences homologous to the human X- and Y-borne zinc finger protein genes (ZFX/Y) are autosomal in monotreme mammals. Genomics. 1993 Feb;15(2):317–322. doi: 10.1006/geno.1993.1063. [DOI] [PubMed] [Google Scholar]
- Whitfield L. S., Lovell-Badge R., Goodfellow P. N. Rapid sequence evolution of the mammalian sex-determining gene SRY. Nature. 1993 Aug 19;364(6439):713–715. doi: 10.1038/364713a0. [DOI] [PubMed] [Google Scholar]
- Yen P. H., Marsh B., Allen E., Tsai S. P., Ellison J., Connolly L., Neiswanger K., Shapiro L. J. The human X-linked steroid sulfatase gene and a Y-encoded pseudogene: evidence for an inversion of the Y chromosome during primate evolution. Cell. 1988 Dec 23;55(6):1123–1135. doi: 10.1016/0092-8674(88)90257-7. [DOI] [PubMed] [Google Scholar]