Abstract
Human Rhinovirus (HRV) is commonly associated with loss of asthma symptom control requiring escalation of care and emergency room visits in many patients. While the association is clear, the mechanisms behind HRV-induced asthma exacerbations remain uncertain. Immune dysregulation via aberrant immune responses, both deficient and exaggerated, have been proposed as mechanisms for HRV-induced exacerbations of asthma. Epithelial-derived innate immune cytokines that bias Th2 responses, including interleukin (IL)-25, IL-33, and thymic stromal lymphopoietin (TSLP), have also been implicated as a means to bridge allergic conditions with asthma exacerbations. In this review, we discuss the literature supporting these positions. We also discuss new and emerging biotherapeutics that may target viral-induced exacerbations of asthma.
Keywords: Asthma Exacerbations, Human Rhinovirus, IL-25, IL-33, TSLP, Biotherapeutics
INTRODUCTION
Asthma remains a significant contributor to morbidity in the United States. It is a chronic heterogeneous disease whose prevalence is ~8% in the US population, and it is characterized by a triad of reversible airflow obstruction, bronchial hyper-responsiveness, and underlying inflammation leading to clinical symptoms1, 2. Patients with asthma typically experience gradual loss of symptom control; however, for many, these changes occur suddenly and are secondary to viral infections. In fact, over 80% of acute asthma exacerbations in children will present in conjunction with a respiratory virus, and the common cold virus, human rhinovirus (HRV), consistently accounts for 60–70% of these virus-associated exacerbations3–5. Yet, the questions remain: why HRV and why asthma?
While many groups have described aberrant immune responses to HRV infection that could be responsible for exacerbations of asthma6–11, the molecular and cellular mechanisms remain poorly understood, and there are currently no consensus opinions regarding the immune responses to HRV in asthmatics. In fact, findings of immune dysregulation, both deficient and exaggerated, are found throughout the literature4, 7, 10, 12, 13, and we will discuss the evidence for each within this review. We will also discuss mechanisms whereby synergy exists between atopic status and viral infection that could cause and/or enhance exacerbations of asthma. Because conventional therapies are only partially effective in the prevention of asthma exacerbations, we will discuss new and emerging treatments that may target viral-induced exacerbations of asthma.
The Case for Immune Dysfunction in Asthmatics
The mechanisms leading to HRV-associated asthma exacerbations are not completely understood, and the complex interactions between viral-induced acute asthma and the immune system provide the backdrop for a myriad of reports that support both deficient and exaggerated immune responses to HRV in this subgroup of patients. With regards to a deficient immune response to infection, proposals suggest the interactions of known asthma pathophysiology and Th2 immune bias in combination with the host response to viral infection may lead to loss of symptom control through deficient innate anti-viral immune responses and abnormal cytokine production14.
Several studies have demonstrated asthmatics have deficient type I interferon (IFN) production in response to HRV and have proposed this as a mechanism for increased susceptibility to and symptoms during HRV infections in asthma subjects13–16. IFN-β, a type I IFN, is produced by lymphocytes, macrophages, fibroblasts, epithelial, and endothelial cells in response to viral infection. Its primary roles are to inhibit viral replication and protect uninfected cells from propagating the infection17. In subjects with asthma, particularly those with severe disease, there have been reports describing deficient IFN-β production in response to HRV infection. Specifically, one study demonstrated that primary bronchial epithelial cells (BECs) from non-atopic, non-asthmatic subjects had a four-fold increase in IFN-β production in response to HRV exposure in vitro when compared to BECs from asthmatic subjects13. Given the relative importance of IFN-β in viral immunity, this study also demonstrated that HRV-infected BECs from subjects with asthma had higher viral loads and cell lysis, leading to increased release of intact viral particles that could infect neighboring cells. Further, both increased viral load and decreased IFN-β production in these cells contributed to an impaired apoptotic response-- a critical part of natural immunity against viruses13. A similar study confirmed that HRV infected BECs from severe therapy resistant asthmatics had impaired IFN-β production and increased viral load when compared to non-asthmatic HRV infected controls15.
Likewise, IFN-λ, a type III IFN also with antiviral properties similar to IFN-β, has been studied in BECs from asthma subjects and controls. One study demonstrated a deficient induction of IFN-λ during HRV infection of BECs from asthma subjects in vitro. As might be expected from a deficient response of IFN-λ, this study also confirmed an increased viral load in these subjects16. Additionally, the study mentioned above of BECs from severe therapy resistant asthmatics confirmed an impaired IFN-λ response to HRV in this group, as well15.
Deficient IFN responses in asthmatics may also help to explain other observed differences in cytokines between healthy controls and subjects with asthma. IFN-β has been implicated in induction and secretion of IL-15, a cytokine produced by mononuclear phagocytic cells and epithelial cells that plays a role as a hematopoietin, chemotactic, and activating factor for natural killer cells17. Therefore, some have hypothesized that decreased IFN-β production in asthmatics would lead to decreased IL-15 production by asthmatic macrophages. One study found that HRV induction of IL-15 by macrophages was impaired in bronchoalveolar lavage (BAL) fluid from asthmatics when compared to healthy controls. Furthermore, the levels of IL-15 in BAL fluid were inversely related to HRV load and airway hyper-responsiveness (AHR) as evidenced by sensitivity to methacholine in subjects with asthma8.
A deficient immune response to HRV leading to increased viral loads and loss of symptom control in asthma is logical, especially given the propensity of those with a Th2 driven disease such as allergic asthma to dampen Th1 type responses. However, HRV is the primary driver of asthma exacerbations in children older than three3, and other viruses, which we know infect these children, do not cause similar symptoms. This supports the argument that the deficient response in asthmatics must be HRV specific. In addition, in vivo studies of HRV loads in asthmatics do not suggest any differences between those with asthma and those with cold symptoms18. Other studies suggest exaggerated immune responses to HRV, and we will discuss these in the next section.
The Case Against Deficient Anti-viral Responses in Asthmatics
Many studies have found exaggerated immune responses to viral infection in asthmatics, and, often, these authors argue that a robust immune response to viral infection leads to acute loss of symptom control in asthma. Several of these studies point to secondary markers including evaluation of viral loads between subjects with asthma and without, finding similar levels and suggesting an intact immune response. Others show exaggerated responses of specific cytokines implicated in the process of viral innate immunity.
If patients with asthma have a deficient anti-viral response to HRV, one might expect them to also have higher viral loads, a finding that is seen in many in vitro studies as shown above. However, other studies looking at susceptibility to HRV and viral load in asthmatics versus healthy controls in vivo have found evidence of similar viral loads between these groups. For example, it has been demonstrated that nasal washes from children who have asthma and are naturally infected with HRV show no difference in viral load when compared to HRV infected non-asthmatics18. Importantly, these patients were seen in the emergency department with asthma exacerbations and/or cold symptoms, and the authors could not predict when the cold symptoms first started or when the subjects were initially exposed to the virus. It further stands to reason that any control subject seen in the emergency department with cold symptoms alone must have severe symptoms, and this information may account for the similarities seen in viral loads between the two groups. However, the same group subsequently controlled for the timing of symptoms by performing experimental infections with HRV16 in adult asthmatics and controls. Viral loads did not differ between those with and without asthma18. Another study performed in Argentina on subjects with asthma and upper respiratory symptoms either with or without wheezing also showed similar viral loads between these groups. These studies support the hypothesis that the inflammatory response to HRV in asthmatics in vivo is intact leading to sufficient viral restriction and killing10.
And what about the differential cytokine responses to HRV observed in asthma subjects? Despite the previous data suggesting deficient viral-induced IFN responses in asthma subjects, many other studies have been shown IFN levels to be the same or even exaggerated in asthmatics14. In the previously mentioned study from Argentina, researchers evaluated nasal wash samples from subjects with asthma and upper respiratory infections with and without wheezing. Besides viral load, they also measured levels of IFN-λ1, finding no significant difference in IFN-λ1 levels between asthmatic children with and without wheezing10. This suggests that IFN-λ1 does not play a role in the pathophysiology of wheezing during asthma exacerbations. Another study arguing against deficient cytokine responses to HRV in asthmatic patients compared IFN-β production in nasal and bronchial epithelial cell cultures from asthmatics and healthy controls. These researchers also found no significant difference in IFN-β expression or susceptibility of the BECs to HRV infection between patients with asthma compared to healthy controls19.
The literature clearly has conflicting views on whether or not immune dysfunction plays a role in HRV and asthma exacerbations. The paradigm of immune dysfunction in asthmatics is further complicated by the presence of multiple murine studies predicting higher viral loads and deficient immune responses in mouse models of asthma and HRV infection. Importantly, the mouse is not a primary host for many human rhinovirus, as the specific receptor required for infection of cells, Intercellular Adhesion Molecule (ICAM), is different in mice. Further more, over the years, HRV has evolved to evade innate immune responses, specifically the retinoic acid-inducible gene-I receptors (RIG-I) and melanoma differentiation-associated protein receptor-5 (MDA5) pathway, in human hosts, despite the diagnosis of asthma. HRV is known to have a protease (3C), which subverts mitochondrial antiviral-signaling protein (MAVS), a protein important in signaling of RIG-I and MDA5, subsequently dampening IFN susceptible genes20. In the mouse, this does not occur, which leads to inherently different immune responses within this model21. Taken together, the data suggest the need for improved ex vivo models and more in vivo studies in humans, which will provide more clues concerning the immune response of asthma subjects to HRV and the risk for exacerbation attributed to this virus.
Synergy Between Allergy and Infection
As described above, the literature is riddled with differing opinions regarding the exaggerated or deficient innate immune responses to HRV in asthmatics. An alternative explanation is derived from the observation that HRV-associated asthma exacerbations are linked directly to the increased expression of a type 2 cytokine signature (IL-4, -5, and -13)9, 22. In studies performed in children from Costa Rica with asthma exacerbations, the risk of wheeze substantially increased if the child was both HRV positive and had high-titer specific IgE to dust mite, implying a link between viral infections and allergic disease5. Other studies confirm this observation and even go a step further to posit evidence of atopy at age 1 or 3 in conjunction with childhood HRV infection defines those who will develop persistent wheezing at age 623. In another HRV16 experimental challenge model using methacholine as a surrogate for exacerbations, only those patients with high total IgE (> 371 IU/mL) had evidence of increased methacholine sensitivity, suggesting a link between high total IgE and AHR after infection11.
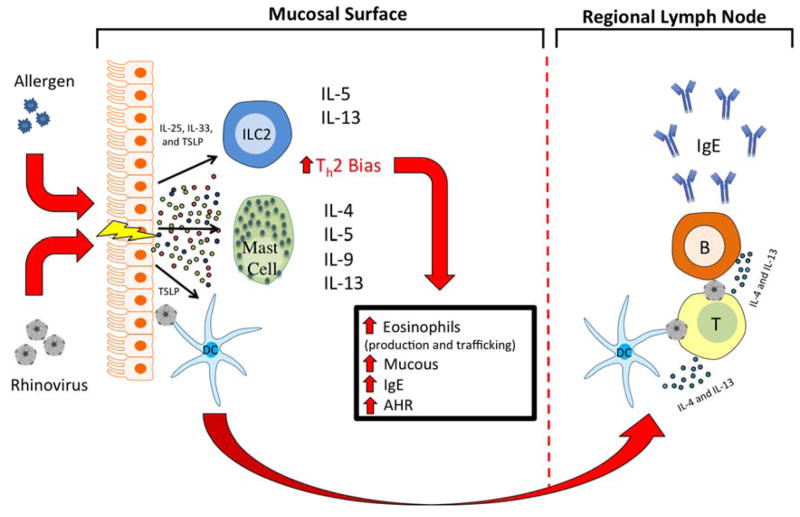
Allergic or infectious inflammation begins with exposure at the epithelial cell surface of the nose, lung, GI tract, and/or skin, triggering a cascade of inflammatory signals. Recent developments in cytokine biology have increasingly emphasized the importance of epithelial-derived cytokines in creating the milieu that promotes the evolution of a Th2 immune response24. In particular, three cytokines, IL-25, IL-33, and thymic stromal lymphopoietin (TSLP), when produced by inflamed epithelium, drive Th2 immune deviation25–27 specifically through their interactions with mast cells, innate lymphoid type 2 cells (ILC2), and naïve T cells. Further, dendritic cells exposed to TSLP at the epithelial surface will travel to regional lymph nodes where the Th2 bias will propagate to naïve T cells, expanding the allergic phenotype (Figure 1).
Figure 1. Allergen exposure and viral infections of the epithelium lead to production of IL-25, IL-33, and TSLP in predisposed asthmatics.
Activation of ILC2 and mast cells generates Th2 signature cytokines, including IL-4, IL-5, IL-9, and IL-13, leading to exacerbations of asthma via increased mucous production and airway hyper-responsiveness (AHR). Dendritic cells exposed to antigen (HRV) and TSLP at the epithelial surface travel to regional lymph nodes where they present the antigen to naïve T cells, subsequently skewing these cells to a Th2 response and leading to class switch and production of IgE by B cells.
IL-25
IL-25 (IL-17E) is a member of the IL-17 family of cytokines, but because of its unique spectrum of activities it has now been given distinct nomenclature. This cytokine is made primarily by epithelial cells and fibroblasts, but other sources include mast cells, eosinophils and basophils. Unlike other members of the IL-17 family, which promote neutrophilic inflammation, IL-25 induces signature allergic cytokine (IL-4, IL-5, IL-9, and IL-13) release from memory Th2 cells, basophils, and ILC2s. IL-25 has been linked to allergic inflammation by its essential role in enhancing the early Th2 response of IL-13 dependent pathways, which contributes to AHR28. Further, IL-25 increases expression of chemokines, CCL5 (RANTES) and CCL11 (eotaxin 1), which contribute to the homing of eosinophils to the lungs and other tissues 25.
The Evidence in Allergy, Asthma, and Infection
Data regarding the importance of epithelial-derived cytokines in the generation of allergic responses has rapidly expanded over the last several years. As mentioned above, several studies have shown that IL-25 stimulates ILC2 cells to secrete IL-5 and IL-13 promoting eosinophilia, increased mucus production, and, ultimately, AHR29, 30. IL-25 is implicated in AHR from studies of allergic sensitized mice treated with methacholine showing that antibody blockade of IL-25 receptor inhibits IL-13 production and eliminates AHR28. Furthermore, a study of 43 recently diagnosed asthma subjects demonstrates levels of IL-25 in the serum correlate with higher total IgE levels31. These data suggest that atopic patients can be divided into subgroups based on amounts of IL-25 (IL-25-high or IL-25-low) that may serve to target individuals for consideration of specific allergic disease therapies.
The most compelling evidence to date for the importance of IL-25, HRV infection, and asthma exacerbations lies within a recent study by Beale, et al. In this study, bronchoalveolar lavage (BAL) samples of allergic asthmatics and non-asthmatics experimentally infected with HRV16 were obtained and measured for the presence of IL-25. Subjects with allergic asthma had higher levels of IL-25 in BAL fluid than control subjects. Further, mouse models of asthma were employed to evaluate HRV infection and expression of IL-25, showing that blockade of IL-25 receptor during infection led to diminished Th2 responses32. Though more data is required, this study suggests that HRV amplifies the IL-25 response and that blockade of IL-25 may lead to prevention of exacerbations of asthma.
IL-33
IL-33, a member of the IL-1 superfamily, serves as the ligand for a heterodimeric receptor complex consisting of ST2 and IL-1R accessory protein33. Similar to other members of the IL-1 superfamily, IL-33 is generated in a pro-form that can be cleaved34. Most members of this family require caspase cleavage for activation; however, evidence suggests that alteration of IL-33 by caspases leaves this cytokine inactivated35.
The Evidence in Allergy, Asthma, and Infection
IL-33 has a vital role in the induction and effector phases of type 2 immune responses, and, as such, it is important in many allergic diseases including asthma, atopic dermatitis, and allergic rhinitis33. Exposure to airborne allergens and respiratory viruses causes damage to the pulmonary epithelium and/or activation of pattern recognition receptors leading to the release of IL-33 from epithelial cells in the airways36, 37. IL-33 drives allergen sensitization via effects on airway dendritic cells and ILC2s leading to development of Th2 signature cytokines and the pathology associated with this allergic response38. Further, genetic polymorphisms of IL-33 near the IL1RL1 locus are strongly linked to asthma, suggesting atopic individuals may be genetically predisposed to secrete more IL-33, especially during allergen challenge39. In a recent study by Jackson and colleagues, experimental HRV inoculation and BAL sampling of human subjects revealed induction of IL-4, IL-5, IL-13, and IL-33 in the airways of asthmatics. Peripheral blood T cells and ILC2 cells from the same subjects cultured with the supernatants of HRV-infected BECs also induced these cytokines, and this process was entirely dependent upon IL-3340.
TSLP
Thymic stromal lymphopoietin (TSLP), another epithelial-derived cytokine, is significantly elevated in those with asthma and allergic diseases. Chemicals, viruses, and allergens are implicated as stimuli for inducing TSLP production in inflamed tissue. TSLP is expressed by epithelial cells of the skin, gut and lung and primes resident DCs to promote Th2 cytokine production by their subsequently engaged effector T cells. TSLP receptors are primarily expressed by DCs, but their expression on mast cells also promotes allergic responses.
The Evidence in Allergy, Asthma, and Infection
In mice, evidence suggests that TSLP is capable of directly stimulating naïve CD4+ T helper cells to differentiate to a Th2 phenotype with increased production of IL-4, IL-5, and IL-13 41. In situ hybridization techniques have been employed in human studies showing increased TSLP in the mucosal and submucosal layer of subjects with asthma that is not observed in individuals without this disease42. Elevated levels of TSLP in asthma subjects also positively correlate with airway obstruction, a characteristic that ultimately leads to difficulty in breathing 42–44.
In a study of nasal epithelial cells, TSLP mRNA expression was significantly enhanced by stimulation of the Toll-like Receptor 3 ligand, a receptor important in recognition of double stranded RNAs (dsRNAs) that occur during viral replication45. Further, the same group showed BECs stimulated with IL-4 and dsRNAs enhance production of Th2 cytokines in mast cells. They also demonstrated anti-TSLP antibodies suppressed this effect on mast cells if BECs were present; however, in the absence of BECs, IL-4 and dsRNAs had no stimulatory effect on mast cells with or without TSLP blockade46. Taken together, these data support that HRV enhances production and secretion of TSLP, providing more evidence of allergic and infectious synergy in the asthmatic airway.
Translating the Science to Novel treatments in Viral-induced Asthma Exacerbations
The studies surrounding the cellular mechanisms by which HRV causes asthma exacerbations have translated to novel and potential treatments that one must consider during interventions for HRV-induced asthma exacerbations. HRV-specific vaccination, enhancement of innate immune responses via inhaled supplementation with IFN-β, and monoclonal antibodies to inflammatory cytokines are presently in development as possible targets to improve outcomes through prevention of viral-induced exacerbations of asthma.
Emerging research has shown that a vaccine against HRV could be beneficial to asthmatics. However, given that there are more than 100 serotypes of HRV, this is a difficult endeavor. Importantly, evidence suggests that antibody responses to HRV are critical in diminishing symptoms, increasing the appeal of a vaccine47. Similar to development of the flu vaccine, researchers have identified highly conserved areas of HRV proteins across multiple serotypes in hopes of creating a vaccine that produces cross-reactivity between all or many serotypes. One study has shown that by identifying a conserved area of the HRV VP0 capsid protein, it is possible to create a vaccine that elicits a response that is effective against multiple HRV species. In this study, mice that had been given a vaccine containing a recombinant VP0 combined with a Th1 promoting adjuvant showed no clinical signs of disease after intranasal challenge with a heterologous HRV. Vaccinated mice were also able to clear the virus more rapidly than those that had not been immunized48. Alternatively, other regions within the HRV capsid structure have also been targeted, including the VP1 protein, which has been shown to induce cross-serotype neutralizing antibody production in mice49. Though a HRV vaccine has not yet been studied in a human model, these data provide promise to the idea that a subunit vaccine could provide cross-reactivity to the hundreds of serotypes of HRV, rather than having to vaccinate against every strain. Such a vaccine could significantly benefit patients with asthma by decreasing the morbidity of viral-induced exacerbations of asthma.
In addition to research aimed at preventing HRV-induced asthma exacerbations, other research has focused on enhancement of innate immune responses via administration of biologic anti-viral agents such as IFN-β. As mentioned previously, many studies question the IFN-β response to HRV in asthma subjects and suggest it is deficient. Even if IFN responses are adequate in asthma subjects, a bolstered response during the infection with exogenous IFN could reduce viral loads and improve symptoms, leading to prevention of exacerbations of asthma. Moreover, daily administration of IFN during the viral season could create a state of enhanced resistance to infection. These ideas have prompted the hypothesis that treatment with inhaled IFN-β could be beneficial, reducing viral exacerbations of asthma13. One randomized, double-blind, placebo-controlled study of asthmatics on inhaled corticosteroids has shown treatment with inhaled IFN-β within 24 hours of the onset of cold symptoms has no significant effect on asthma symptom control, as measured by the Asthma Control Questionnaire. However, it does decrease the need for additional treatment, such as antibiotics and corticosteroids, and enhanced the innate immune responses, as evidenced by decreased levels of inflammatory cytokines and reduced viral load. Additionally, a post hoc analysis of more difficult to treat asthma patients (defined as those meeting criteria for British Thoracic Society steps 4–5) showed that administration of inhaled IFN-β within 24 hours of upper respiratory illness symptoms does have a protective effect against exacerbations50.
Many believe the combination of allergy and infection leads to alterations of the immune response to HRV, leading to exacerbations. Therefore, it stands to reason that if allergic responses are muted in the host by directed treatments against IgE, symptoms associated viral-induced exacerbations of asthma may improve. The monoclonal antibody, omalizumab, targets the high-affinity receptor-binding site on IgE, effectively removing much of the free IgE from circulation. In a multicenter study of inner-city patients with persistent allergic asthma, omalizumab led to a reduction in days with asthma symptoms, reduction in exacerbations and hospitalizations, and reduced need for inhaled corticosteroids to maintain control. Additionally, omalizumab nearly eliminated the seasonal peaks of asthma exacerbations, both in the fall and spring, that were seen in the placebo group. Viruses were detected in similar numbers from both the treatment and control populations throughout the year, but the treatment group had a significant decrease in exacerbations, supporting the association discussed between viral infection and allergen responses during asthma exacerbations51.
Recently, a monoclonal antibody to TSLP has been evaluated. In these studies, allergen challenges have been used to study whether the antibody, AMG157, attenuates the allergic response in asthmatics and improves symptoms. This double-blind, placebo-controlled study of patients with mild allergic asthma (defined as positive skin-prick, FEV1 >70%, and AHR to methacholine) shows that those who received AMG 157 had attenuated inflammatory responses after challenge as evidenced by decreased blood and sputum eosinophils, as well as lower fraction of exhaled nitric oxide as compared to those who did not receive the drug52. Human monoclonal antibodies to IL-25 and IL-33 are not currently available. However, recent data support improved symptoms during HRV infection in mice when these cytokines are blocked. As previously noted, Beale, et al. has shown that blockade of the IL-25 receptor in mice correlated with decreased inflammatory responses during HRV infections32. Further, the same group suggests that antibodies to IL-33 diminish type 2 cytokine production in asthmatic BECs during HRV infection40. Taken together, these biological targets remain viable candidates for future treatments of viral-induced asthma.
Conclusion
HRV-induced exacerbations of asthma are important enhancers of morbidity in a disease that already causes significant disturbances to quality of life in patients. The efficiency of the innate immune system, either as deficient or enhanced, in asthmatics has been evaluated, and the evidence is not consistent. However, these studies may be identifying specific sub-groups of patients that are at risk for developing HRV-induced asthma exacerbations, and the unique cellular immune factors present in each patient may drive these exacerbations. As science progresses, clinicians will stop administering “one size fits all” treatments but instead recognize the need for personalized treatment regimes addressing the unique mechanisms of each asthmatic patient.
The bridge between allergy and infection is far from complete, but the groundwork has been laid with regards to mechanisms involving epithelial-derived cytokines and exacerbations of asthma. As shown, IL-25, IL-33, and TSLP are important contributors to allergy, asthma, and infection with viruses, and they are being considered as targets for biotherapeutics. Other interventions, such as vaccines, hold promise for prevention of asthma exacerbations in the future; however, until human studies are performed, the efficacy of these interventions in prevention of viral-induced exacerbations remains unknown. While the future is bright regarding prevention of HRV-associated asthma exacerbations, the history of incompatibility that exists between HRV and asthma continues to repeat itself in many of our patients presently.
Acknowledgments
The authors would like to thank Drs. Larry Borish, John Steinke, and Stacie Jones for their gracious edits of this manuscript.
Footnotes
Conflicts of interest: Dr. Kennedy has received asthma research support through the NIH (UL1TR000039, KL2TR000063, P20GM103625) and the American Academy of Allergy, Asthma, and Immunology (ARTrust mini-grant). The rest of the authors declare no conflicts of interest.
Contributor Information
Catherine Hammond, Email: CMHammond@uams.edu.
Megan Kurten, Email: MAKurten@uams.edu.
References
*Of Importance
** Of Major Importance
- 1.National Asthma E, Prevention P. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120:S94–138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 2.Blackwell DLLJ, Clarke TC. Summary health statistics for U.S. adults: National Health Interview Survey, 2012. National Center for Health Statistics. Vital Health Stat. 2014:10. [PubMed] [Google Scholar]
- 3.Heymann PW, Carper HT, Murphy DD, Platts-Mills TA, Patrie J, McLaughlin AP, et al. Viral infections in relation to age, atopy, and season of admission among children hospitalized for wheezing. The Journal of allergy and clinical immunology. 2004;114:239–47. doi: 10.1016/j.jaci.2004.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McMeen VSM, Gern J, Carper H, Murphy D, Vrtis R, Platts-Mills T, Heymann PW. Viral load assessments of rhinovirus (RV) by quantitative RT-PCR in nasal washes from children treated in the emergency department (ED) for Asthma. J Allergy Clin Immunol. 2008:121. [Google Scholar]
- 5*.Soto-Quiros M, Avila L, Platts-Mills TA, Hunt JF, Erdman DD, Carper H, et al. High titers of IgE antibody to dust mite allergen and risk for wheezing among asthmatic children infected with rhinovirus. The Journal of allergy and clinical immunology. 2012;129:1499–505. e5. doi: 10.1016/j.jaci.2012.03.040. Provides evidence that viral infection in an atopic, asthmatic host leads to exacerbations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avila PC, Abisheganaden JA, Wong H, Liu J, Yagi S, Schnurr D, et al. Effects of allergic inflammation of the nasal mucosa on the severity of rhinovirus 16 cold. The Journal of allergy and clinical immunology. 2000;105:923–32. doi: 10.1067/mai.2000.106214. [DOI] [PubMed] [Google Scholar]
- 7.Brooks GD, Buchta KA, Swenson CA, Gern JE, Busse WW. Rhinovirus-induced interferon-gamma and airway responsiveness in asthma. American journal of respiratory and critical care medicine. 2003;168:1091–4. doi: 10.1164/rccm.200306-737OC. [DOI] [PubMed] [Google Scholar]
- 8*.Laza-Stanca V, Message SD, Edwards MR, Parker HL, Zdrenghea MT, Kebadze T, et al. The Role of IL-15 Deficiency in the Pathogenesis of Virus-Induced Asthma Exacerbations. PLoS pathogens. 2011;7:e1002114. doi: 10.1371/journal.ppat.1002114. Determines that IL-15 is deficient at baseline in asthma subjects during experiment RV challenge, and this deficiency correlates with higher viral loads. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Message SD, Laza-Stanca V, Mallia P, Parker HL, Zhu J, Kebadze T, et al. Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:13562–7. doi: 10.1073/pnas.0804181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10*.Miller EK, Hernandez JZ, Wimmenauer V, Shepherd BE, Hijano D, Libster R, et al. A mechanistic role for type III IFN-lambda1 in asthma exacerbations mediated by human rhinoviruses. American journal of respiratory and critical care medicine. 2012;185:508–16. doi: 10.1164/rccm.201108-1462OC. Suggests that IFN-λ1 are comparable between groups of asthma subjects with RV-infection who are wheezing and not. Concludes that IFN-λ1 levels are not predictive of exacerbations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zambrano JC, Carper HT, Rakes GP, Patrie J, Murphy DD, Platts-Mills TA, et al. Experimental rhinovirus challenges in adults with mild asthma: response to infection in relation to IgE. The Journal of allergy and clinical immunology. 2003;111:1008–16. doi: 10.1067/mai.2003.1396. [DOI] [PubMed] [Google Scholar]
- 12.Wark PA, Bucchieri F, Johnston SL, Gibson PG, Hamilton L, Mimica J, et al. IFN-gamma-induced protein 10 is a novel biomarker of rhinovirus-induced asthma exacerbations. The Journal of allergy and clinical immunology. 2007;120:586–93. doi: 10.1016/j.jaci.2007.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wark PA, Johnston SL, Bucchieri F, Powell R, Puddicombe S, Laza-Stanca V, et al. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. The Journal of experimental medicine. 2005;201:937–47. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14**.Gavala ML, Bashir H, Gern JE. Virus/allergen interactions in asthma. Curr Allergy Asthma Rep. 2013;13:298–307. doi: 10.1007/s11882-013-0344-1. A recent detailed review on the interactions between viral infection and atopic status. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15*.Edwards MR, Regamey N, Vareille M, Kieninger E, Gupta A, Shoemark A, et al. Impaired innate interferon induction in severe therapy resistant atopic asthmatic children. Mucosal Immunol. 2013;6:797–806. doi: 10.1038/mi.2012.118. Provides data showing a deficient IFN response RV infection in severe therapy resistant atopic asthmatics and suggests that this may be the result of lower levels of TLRs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Contoli M, Message SD, Laza-Stanca V, Edwards MR, Wark PA, Bartlett NW, et al. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med. 2006;12:1023–6. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]
- 17.Commins SP, Borish L, Steinke JW. Immunologic messenger molecules: cytokines, interferons, and chemokines. J Allergy Clin Immunol. 2010;125:S53–72. doi: 10.1016/j.jaci.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 18**.Kennedy JL, Shaker M, McMeen V, Gern J, Carper H, Murphy D, et al. Comparison of viral load in individuals with and without asthma during infections with rhinovirus. Am J Respir Crit Care Med. 2014;189:532–9. doi: 10.1164/rccm.201310-1767OC. Offers evidence that viral loads are similar in vivo between those with RV-induced asthma exacerbations and non-atopic controls with cold symptoms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopez-Souza N, Favoreto S, Wong H, Ward T, Yagi S, Schnurr D, et al. In vitro susceptibility to rhinovirus infection is greater for bronchial than for nasal airway epithelial cells in human subjects. J Allergy Clin Immunol. 2009;123:1384–90. e2. doi: 10.1016/j.jaci.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drahos J, Racaniello VR. Cleavage of IPS-1 in cells infected with human rhinovirus. J Virol. 2009;83:11581–7. doi: 10.1128/JVI.01490-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rasmussen AL, Racaniello VR. Selection of rhinovirus 1A variants adapted for growth in mouse lung epithelial cells. Virology. 2011;420:82–8. doi: 10.1016/j.virol.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jartti T, Paul-Anttila M, Lehtinen P, Parikka V, Vuorinen T, Simell O, et al. Systemic T-helper and T-regulatory cell type cytokine responses in rhinovirus vs. respiratory syncytial virus induced early wheezing: an observational study. Respiratory research. 2009;10:85. doi: 10.1186/1465-9921-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. American journal of respiratory and critical care medicine. 2008;178:667–72. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wisniewski JA, Borish L. Novel cytokines and cytokine-producing T cells in allergic disorders. Allergy and asthma proceedings : the official journal of regional and state allergy societies. 2011;32:83–94. doi: 10.2500/aap.2011.32.3428. [DOI] [PubMed] [Google Scholar]
- 25.Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–95. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 26.Louten J, Rankin AL, Li Y, Murphy EE, Beaumont M, Moon C, et al. Endogenous IL-33 enhances Th2 cytokine production and T-cell responses during allergic airway inflammation. International immunology. 2011;23:307–15. doi: 10.1093/intimm/dxr006. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Zhou X, Zhou B. DC-derived TSLP promotes Th2 polarization in LPS-primed allergic airway inflammation. European journal of immunology. 2012;42:1735–43. doi: 10.1002/eji.201142123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ballantyne SJ, Barlow JL, Jolin HE, Nath P, Williams AS, Chung KF, et al. Blocking IL-25 prevents airway hyperresponsiveness in allergic asthma. J Allergy Clin Immunol. 2007;120:1324–31. doi: 10.1016/j.jaci.2007.07.051. [DOI] [PubMed] [Google Scholar]
- 29.Lambrecht BN, Hammad H. Allergens and the airway epithelium response: Gateway to allergic sensitization. J Allergy Clin Immunol. 2014;134:499–507. doi: 10.1016/j.jaci.2014.06.036. [DOI] [PubMed] [Google Scholar]
- 30.Licona-Limon P, Kim LK, Palm NW, Flavell RA. TH2, allergy and group 2 innate lymphoid cells. Nat Immunol. 2013;14:536–42. doi: 10.1038/ni.2617. [DOI] [PubMed] [Google Scholar]
- 31.Cheng D, Xue Z, Yi L, Shi H, Zhang K, Huo X, et al. Epithelial interleukin-25 is a key mediator in Th2-high, corticosteroid-responsive asthma. Am J Respir Crit Care Med. 2014;190:639–48. doi: 10.1164/rccm.201403-0505OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32**.Beale J, Jayaraman A, Jackson DJ, Macintyre JD, Edwards MR, Walton RP, et al. Rhinovirus-induced IL-25 in asthma exacerbation drives type 2 immunity and allergic pulmonary inflammation. Sci Transl Med. 2014;6:256ra134. doi: 10.1126/scitranslmed.3009124. Evaluates the role of IL-25 in atopic asthmatic subjects during RV-infection and shows increased levels in asthmatics compared to controls. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohno T, Morita H, Arae K, Matsumoto K, Nakae S. Interleukin-33 in allergy. Allergy. 2012;67:1203–14. doi: 10.1111/all.12004. [DOI] [PubMed] [Google Scholar]
- 34**.Lefrancais E, Roga S, Gautier V, Gonzalez-de-Peredo A, Monsarrat B, Girard JP, et al. IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:1673–8. doi: 10.1073/pnas.1115884109. Detects IL-33 that is more bioactive after exposure to neutrophil mediators, suggesting a link between neutrophils and innate epithelial cell immune responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cayrol C, Girard JP. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:9021–6. doi: 10.1073/pnas.0812690106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Le Goffic R, Arshad MI, Rauch M, L’Helgoualc’h A, Delmas B, Piquet-Pellorce C, et al. Infection with influenza virus induces IL-33 in murine lungs. American journal of respiratory cell and molecular biology. 2011;45:1125–32. doi: 10.1165/rcmb.2010-0516OC. [DOI] [PubMed] [Google Scholar]
- 37.Polumuri SK, Jayakar GG, Shirey KA, Roberts ZJ, Perkins DJ, Pitha PM, et al. Transcriptional regulation of murine IL-33 by TLR and non-TLR agonists. Journal of immunology. 2012;189:50–60. doi: 10.4049/jimmunol.1003554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borish L, Steinke JW. Interleukin-33 in asthma: how big of a role does it play? Current allergy and asthma reports. 2011;11:7–11. doi: 10.1007/s11882-010-0153-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, Heath S, et al. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363:1211–21. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40**.Jackson DJ, Makrinioti H, Rana BM, Shamji BW, Trujillo-Torralbo MB, Footitt J, et al. IL-33-dependent Type 2 Inflammation During Rhinovirus-induced Asthma Exacerbations In Vivo. Am J Respir Crit Care Med. 2014 doi: 10.1164/rccm.201406-1039OC. Shows increased levels of IL-33 in the lungs of asthma subjects during experimental RV infection as compared to non-atopic controls. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Omori M, Ziegler S. Induction of IL-4 expression in CD4(+) T cells by thymic stromal lymphopoietin. J Immunol. 2007;178:1396–404. doi: 10.4049/jimmunol.178.3.1396. [DOI] [PubMed] [Google Scholar]
- 42.Ying S, O’Connor B, Ratoff J, Meng Q, Mallett K, Cousins D, et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol. 2005;174:8183–90. doi: 10.4049/jimmunol.174.12.8183. [DOI] [PubMed] [Google Scholar]
- 43*.Shikotra A, Choy DF, Ohri CM, Doran E, Butler C, Hargadon B, et al. Increased expression of immunoreactive thymic stromal lymphopoietin in patients with severe asthma. J Allergy Clin Immunol. 2012;129:104–11. e1–9. doi: 10.1016/j.jaci.2011.08.031. [DOI] [PubMed] [Google Scholar]
- 44.Xu G, Zhang L, Wang DY, Xu R, Liu Z, Han DM, et al. Opposing roles of IL-17A and IL-25 in the regulation of TSLP production in human nasal epithelial cells. Allergy. 2010;65:581–9. doi: 10.1111/j.1398-9995.2009.02252.x. [DOI] [PubMed] [Google Scholar]
- 45.Kato A, Favoreto S, Jr, Avila PC, Schleimer RP. TLR3- and Th2 cytokine-dependent production of thymic stromal lymphopoietin in human airway epithelial cells. J Immunol. 2007;179:1080–7. doi: 10.4049/jimmunol.179.2.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nagarkar DR, Poposki JA, Comeau MR, Biyasheva A, Avila PC, Schleimer RP, et al. Airway epithelial cells activate TH2 cytokine production in mast cells through IL-1 and thymic stromal lymphopoietin. J Allergy Clin Immunol. 2012;130:225–32. e4. doi: 10.1016/j.jaci.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alper CM, Doyle WJ, Skoner DP, Buchman CA, Cohen S, Gwaltney JM. Prechallenge antibodies moderate disease expression in adults experimentally exposed to rhinovirus strain hanks. Clin Infect Dis. 1998;27:119–28. doi: 10.1086/514634. [DOI] [PubMed] [Google Scholar]
- 48*.Glanville N, McLean GR, Guy B, Lecouturier V, Berry C, Girerd Y, et al. Cross-serotype immunity induced by immunization with a conserved rhinovirus capsid protein. PLoS Pathog. 2013;9:e1003669. doi: 10.1371/journal.ppat.1003669. Details murine experience with cross-serotype immunity provided by immunization with VP0, a capsid protein with high homology across RV serotypes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McLean GR, Walton RP, Shetty S, Peel TJ, Paktiawal N, Kebadze T, et al. Rhinovirus infections and immunisation induce cross-serotype reactive antibodies to VP1. Antiviral Res. 2012;95:193–201. doi: 10.1016/j.antiviral.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 50**.Djukanovic R, Harrison T, Johnston SL, Gabbay F, Wark P, Thomson NC, et al. The effect of inhaled IFN-beta on worsening of asthma symptoms caused by viral infections. A randomized trial. Am J Respir Crit Care Med. 2014;190:145–54. doi: 10.1164/rccm.201312-2235OC. Suggests that exogenous IFN-b may be effective in prevention of HRV-induced exacerbations of asthma in difficult-to-treat patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011;364:1005–15. doi: 10.1056/NEJMoa1009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52**.Gauvreau GM, O’Byrne PM, Boulet LP, Wang Y, Cockcroft D, Bigler J, et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N Engl J Med. 2014;370:2102–10. doi: 10.1056/NEJMoa1402895. Double-blind, placebo-controlled study of a monoclonal antibody against TSLP (AMG157) showing improved allergen-induced bronchoconstriction in asthmatics before and after allergen challenge. [DOI] [PubMed] [Google Scholar]