Abstract
Background
Angiogenic cytokines fms-like tyrosine kinase-1(sFlt-1) and placental growth factor (PlGF) are associated with increased risk for cardiovascular disease (CVD) in the general population. In this study we examine the association between these vascular endothelial factors and atherosclerosis, cardiovascular outcome, and mortality in chronic kidney disease (CKD) patients.
Methods
Serum level of PlGF and sFlt-1 were measured in 301 patients with CKD, who were followed for up to 4 years. Primary outcomes were CV events and all-cause mortality. Carotid-intima media thickness (CIMT) was used as marker of atherosclerosis. Kaplan-Meier survival curves and the Cox proportional hazard model were used to assess the association of biomarkers and clinical outcomes.
Results
Mean (SD) PlGF and sFlt-1 were 5.45 ng/ml (3.76) and 68.6 (28.0) pg/ml, respectively. During the follow up time, 60 patients (19.9%) experienced CV events and 22 patients (7.3%) died. Compared with low PlGF, patients with PlGF above median level had higher CV events (12.7% vs. 27.2%, p=0.002) and mortality (2.0% vs. 12.6%, p < 0.001). The associations of PlGF and sFlt-1 with CV events were not statistically significant in the fully adjusted model. Higher PlGF was associated with greater death risk (HR=5.22, 95%CI: 1.49–18.33, p=0.01), which was robust to adjustment for sFlt-1 and other risk factors. Elevated sFlt-1 level was also an independent predictor of mortality (HR 3.41, 95%CI: 1.49-9.51, p=0.019).
Conclusion
In CKD patients not yet on dialysis, higher serum level of PlGF and sFlt-1 are associated with increased mortality, but not CV events.
Keywords: sFlt, Placental growth factor, vascular endothelial growth factors, survival
1. Introduction
Patients with chronic kidney disease (CKD) are at increased risk of cardiovascular disease (CVD) and mortality compared to the general population.[1] Traditional CV risk factors such as advanced age, diabetes, smoking, and hyperlipidemia are more prevalent in CKD population. However, compelling evidence shows that non-traditional risk factors such as uremic toxins, endotoxemia, malnutrition, and inflammation play pivotal role in CVD, and poor outcome in CKD patients, at least in part by amplifying the risk of atherosclerosis in CKD patients. [2–4]
Vascular endothelial growth factors (VEGF) are a family of endothelial-specific mitogens that have angiogenic properties.[5, 6] Placental growth factor (PlGF) is a member of the VEGF family that binds to the VEGF receptor-1 and mediates angiogenesis and endothelial dysfunction.[7] It was first discovered in the placenta; however, it was later localized to other tissues such as heart and lung. Soluble VEGF receptor 1, also known as soluble fms-like tyrosine kinase-1(sFlt-1), is a splice variant of the VEGF receptor without the transmembrane and intracellular tyrosine kinase domain. It is a potent endogenous antagonist of VEGF and PlGF.[8, 9] PlGF-expression within human atherosclerotic lesions is associated with vascular inflammation, thrombus formation, and plaque destabilization.[10] PlGF and sFlt-1 levels are elevated in patients with coronary artery disease and are predictors of adverse outcome.[11]
There is emerging interest in angiogenic cytokines in the pathogenesis of CVD in patients with CKD.[8] In the present study, we investigated the association of circulating levels of PlGF and sFlt-1 with markers of atherosclerosis, cardiovascular outcome and all-cause mortality in a prospective cohort of CKD patients with longitudinal follow-up up to 4 years.
2. Methods
CARE FOR HOMe study is an ongoing study of CKD patients from outpatient nephrology clinic in Saarland University Hospital. The study was approved by the local Ethics Committee, and all patients signed written informed consent. Patients were included in this study if they had CKD stage 2, 3, or 4, defined as an estimated glomerular filtration rate (eGFR) between 15 and 90 ml/min/1.73m2 according to MDRD equation. Patients younger than 18 years of age, pregnant women (based on self-report), allograft recipients, patient receiving systemic immunosuppressive medication and those with concomitant human immunodeficiency virus infection, clinically apparent infections (defined as C-Reactive Protein (CRP) levels above 50 mg/l, and/or requiring systemic antibiotic therapy), active cancer disease, malignant hematological disorders, and/or acute renal failure (defined as an increase of plasma creatinine ≥50% within four weeks) were excluded from study participation.
A standardized questionnaire was used to record a history of smoking, diabetes mellitus status, current medication intake, and cardiovascular co-morbidities. Furthermore, chart review was done to complete and ascertain co-morbidities. Prevalent CVD was defined as a history of myocardial infarction, coronary artery angioplasty and/or stenting and/or coronary bypass surgery, major stroke, carotid endarterectomy and/or stenting, non-traumatic above the knee amputation, or lower limb artery bypass surgery and/or angioplasty and/or stenting.
Patients were categorized as active smokers if they were current smokers or had stopped smoking < 1 month prior to participation. Patients with self-reported or physician-reported diabetes mellitus, with a fasting blood glucose level of ≥126 mg/dl or with current use of hypoglycemic medication, were categorized as diabetic. Body mass index (BMI) was calculated as weight (kg)/(height (m2)).
All patients were invited annually for follow-up examinations. The combined end points were as described earlier, which is first occurrence of an atherosclerotic event, the time to decompensated heart failure or death from any cause. [12] All patients were followed up until 31 December 2012.
2.1. Laboratory measurements
Blood samples were obtained under standardized conditions after an overnight fast. Within 15 minutes, the samples were centrifuged at 4000 r.p.m. for 5 minutes at room temperature. Supernatants were immediately stored in aliquots at −80 °C until further use. Serum level of intact PTH was measured by second-generation ECLIA (Hoffmann-La Roche, Bale, Switzerland; range of normal values: 15–65 pg/ml), and serum levels of calcium, and phosphate were measured by standard laboratory methods. In all 301 patients, sFlt-1 levels were determined by Quantikine kit (Human sVEGF R1/Flt-1, Cat #: DVR100B, Sensitivity: 1.5-13.3 pg/ml with mean minimum detectable dose of 3.5 pg/ml. CV(%): 2.6 intra-assay precision and 5.5 inter-assay precision). PLGF was measured by sandwich enzyme immunoassay using commercial ELISA kit (R&D Minneapolis, USA). All measurements were done in duplicates. Intra and inter-assay variability were less than 12%.
2.2 Assessment of atherosclerosis
As a marker of systemic vascular atherosclerosis, the intima media thickness of the common carotid arteries was measured (CIMT). With the subject in the supine position and the head slightly extended and turned to the opposite direction, the distal common carotid artery and the carotid bulb were identified with longitudinal scanning. IMT was defined as the distance between the leading edges of the lumen interface and the media-adventitia interface of the far wall. Three representative CIMT measurements were performed on both sides in the far wall of the common carotid arteries at 1.0, 2.0, and 3.0 cm proximal to the bifurcation, and these six CIMT readings were averaged to give the mean common carotid CIMT.
2.3 Statistical analysis
Data management and statistical analysis were performed with SPSS version 17. P values < 0.05 was considered statistically significant. Categorical variables are presented as percentage of patients and were compared using the Chi-2 test. Continuous data are expressed as mean ± standard deviation, or median (quartile 25–75) for variables with skewed distribution. A T-test or Mann-Whitney U test were used to compare mean or median of variables in the two groups of patients with PlGF above vs. below median level. The correlation between continuous variables was assessed by Pearson correlation testing or Spearman’s rho, whichever was appropriate. The most appropriate PlGF transformation for fitting the PlGF vs. eGFR association was investigated based on minimizing the Akaike Information Criterion (AIC).[13] Multivariate linear regression model, with stepwise approach, was used to examine the predictors of PlGF. Variables that were used in the stepwise model included age, sex, eGFR, creatinine, Cystatin C, albuminuria, LDL-C, HDL-C, Triglyceride, WBC, PTH. Cox proportional hazard model, Kaplan-Meier survival curves, and log-rank test were used to assess the association of PlGF and clinical outcomes. First, we used Cox proportional hazard model without adjusting for confounders to examine the hazard ratio (HR) of cardiovascular outcome or mortality in CKD patients with PlGF above median level versus those below the median level as reference group. We used the same approach for association of sFlt-1 and outcome. In model 1, we included both PlGF and sFlt-1 to examine the proportional association of each variable with outcomes. Model 2 was adjusted for age and sex. Model 3 was adjusted for BMI, diabetes, smoking, SBP, and LDL-C as well as age and sex. In model 4, albuminuria and eGFR were also added to the previous adjustors.
3. Results
Mean patient age was 65.7±11.8 years. Sixty one percent of participant were male (n=184), 39% (n=118) had diabetes, 32.7% (n=98) had known cardiovascular disease, and 11.3% (n=34) were smokers. CKD stages 2, 3, and 4 encompassed 16.6% (n=50), 60.8% (n=183), and 22.6% (n=68) of the total cohort, respectively. Mean (SD) of PlGF was 5.45 ng/ml (3.76), ranging from 0.1 to 22.3 ng/ml. Mean (SD) of sFlt-1 was 68.8 pg/ml (28.0), ranging from 19.2 to 201.9 pg/ml (Supplemental Fig. 1A and B).
Table 1 shows the baseline general, demographic, and biochemical characteristics of the study participants in total and in patients above and below median level of PlGF. At baseline, patients with PlGF above median level had lower HDL-C (p= 0.001), higher serum PTH (p=0.021), and higher prevalence of CVD (p= 0.03). There was no significant difference between serum level of PlGF among CKD patients with or without prior history of CVD (6.1±3.8 vs. 5.4±3.7 ng/ml, respectively; p=0.26). Likewise, serum level of PlGF was not different between diabetic and non-diabetic patients (5.7±3.8 vs. 5.3±3.8 ng/ml, respectively; p=0.31), and between patients on statin therapy and those who were not on statin (5.2±3.4 vs. 5.7±4.0 ng/ml, respectively; p=0.19). There was a weak but significant inverse correlation between serum level of PlGF and eGFR (r= −0.13, p=0.022). There was also a trend towards higher level of PlGF in advanced stages of CKD (Supplemental Fig. 2, p for trend=0.019). CIMT was not statistically significantly different across tertiles of PlGF (Supplemental Fig. 3, p for trend=0.139).
Table 1.
Baseline characteristics of 301 individuals in total, and in patients with placental growth factor (PlGF) above verses below median level
| Characteristics |
All participants (n=301) |
PlGF level below median <4.42 pg/ml (n=150) |
PlGF level above median ≥4.42 pg/ml (n=151) |
P value |
|---|---|---|---|---|
| Demographic and clinical data | ||||
| Male (%) | 61.1 | 56.7 | 65.6 | 0.11 |
| Age (years) | 66±12 | 65±12 | 66±11 | 0.5 |
| Diabetes mellitus (%) | 39.2 | 35.3 | 43 | 0.17 |
| CVD (%) | 32.7 | 26.7 | 38.7 | 0.03 |
| Smoker (%) | 11.3 | 8.7 | 13.9 | 0.15 |
| BMI (kg/m2) | 30.2±5.5 | 30.4±6.0 | 30.2±4.9 | 0.76 |
| SBP (mm-Hg) | 154±25 | 155±26 | 153±24 | 0.46 |
| DBP (mm-Hg) | 87±13 | 87±14 | 86±12 | 0.47 |
| Laboratory data | ||||
| sFlt-1 (pg/ml) | 68.6±28.0 | 69.5±28.8 | 67.8±27.1 | 0.60 |
| eGFR (ml/min/1.73 m2) | 43±15 | 45±15 | 43±16 | 0.11 |
| Creatinine (mg/dl) | 1.64±0.67 | 1.56±0.62 | 1.73±0.71 | 0.26 |
| Albuminuria (mg/g) | 0.04 (0.01–0.20) | 0.03 (0.01–0.13) | 0.05 (0.01–0.29) | 0.056 |
| Albumin (g/l) | 43.9±3.3 | 43.6±3.3 | 44.4±3.2 | 0.30 |
| Hemoglobin (g/dl) | 13.4±1.7 | 13.4±1.6 | 13.5±1.8 | 0.82 |
| Leukocytes (1000/ml) | 6.8±1.8 | 6.5±1.7 | 7.2±1.9 | 0.001 |
| Platelets (1000/ml) | 221±61 | 219±57 | 223±65 | 0.53 |
| C Reactive Protein (mg/l) | 2.7 (1.2–5.0) | 2.4 (1.1–4.7) | 2.8 (1.2–5.9) | 0.30 |
| Ferritin (ng/ml) | 140 (79–256) | 128 (73–253) | 151 (92–270) | 0.25 |
| PTH (pg/ml) | 53 (37–88) | 49 (35–74) | 58 (41–95) | 0.021 |
| CIMT (mm) | 0.66±0.14 | 0.65±0.14 | 0.67±0.14 | 0.16 |
| Triglycerides (mg/dl) | 138 (97–204) | 128 (92–185) | 157 (107–221) | 0.73 |
| LDL-C (mg/dl) | 117±35 | 114 ±35 | 119±36 | 0.27 |
| HDL-C (mg/dl) | 51±17 | 54±18 | 47±16 | 0.001 |
PlGF: placental growth factor; sFlt-1: soluble fms-like tyrosine kinase-1; eGFR: estimated glomerular filtration rate; PTH: parathyroid hormone, CIMT: carotid-intima media thickness; LDL-C: Low density lipoprotein cholesterol; HDL-C: High density lipoprotein cholesterol; eGFR was calculated based on 4-parameter MDRD formula
Table 2 shows the correlation coefficients between serum level of PlGF and sFlt-1 with the markers of kidney disease, nutrition, and inflammation. PlGF and sFlt were positively correlated with cystatin C, albuminuria, and pro-BNP, but negatively with eGFR. While examining the association between PlGF and eGFR, the best-fitting transformation was found to have a negative and significant correlation with eGFR (Supplementary Table 1). However, in multivariate regression analysis, only cystatin C, HDL-C, and triglycerides were independent predictors of PlGF (Data not shown). There was no significant correlation between serum PlGF and sFlt-1 (Supplemental Fig. 4).
Table 2.
Bivariate correlations between placental growth factor (PlGF) and soluble fms-like tyrosine kinase-1(sFlt-1) and other related variables
| sFlt-1 | Age | BMI | eGFR (MDRD) | Cystatin C | Albuminuria | Albumin | HDL-C | LDL-C | TG | PTH | WBC | CRP | IMT | Pro-BNP | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PlGF | −0.08 | 0.04 | −0.013 | −0.13 | 0.16 | 0.15* | 0.11 | −0.23 | 0.10 | 0.20* | 0.15* | 0.142 | 0.04 | 0.11 | 0.104* |
| p | 0.19 | 0.44 | 0.08 | 0.022 | 0.007 | 0.009 | 0.052 | <0.001 | 0.073 | 0.001 | 0.01 | 0.014 | 0.48 | 0.07 | 0.072 |
| sFlt-1 | n/a | 0.12 | −0.111 | −0.121 | 0.145 | 0.123* | −0.138 | −0.012 | −0.081 | −0.011 | 0.128* | 0.004 | −0.016 | 0.015 | 0.181* |
| p | n/a | 0.037 | 0.043 | 0.028 | 0.009 | 0.034 | 0.012 | 0.824 | 0.161 | 0.836 | 0.020 | 0.943 | 0.768 | 0.789 | 0.002 |
Non–parametric correlation coefficient (Spearman’s rho)
PlGF: placental growth factor; sFlt-1: soluble vascular endothelial growth factor receptor-1; BMI: body mass index; eGFR: estimated glomerular filtration rate; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; TG: triglyceride; PTH: parathyroid hormone; WBC: white blood cell; CRP: C-reactive protein; IMT: Intimamedia thickness
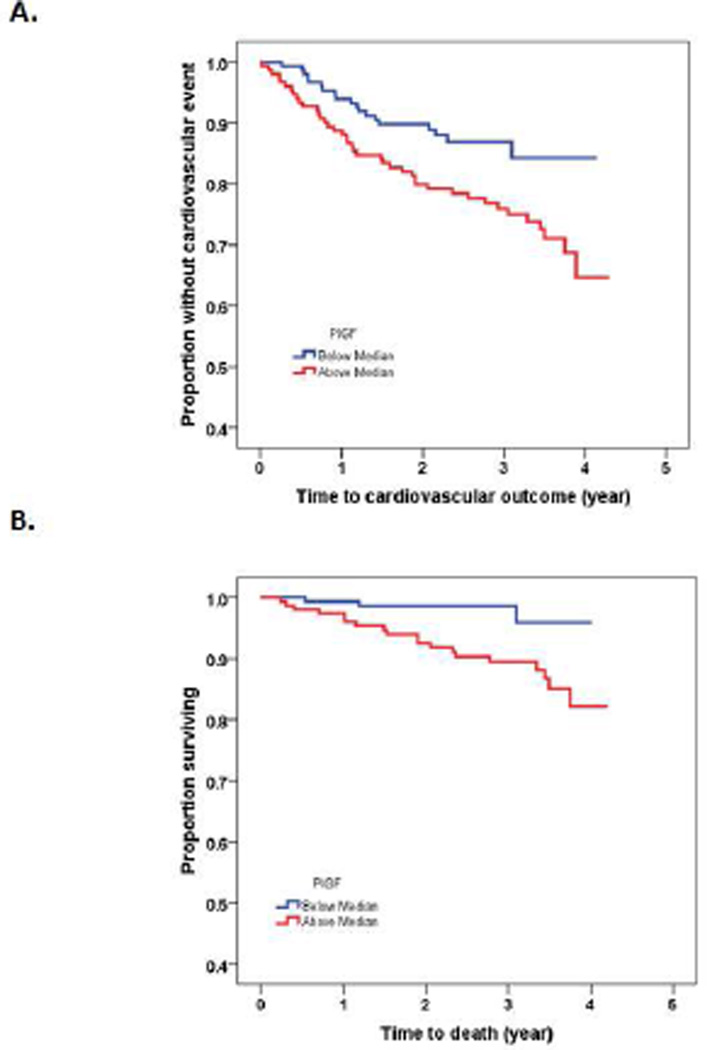
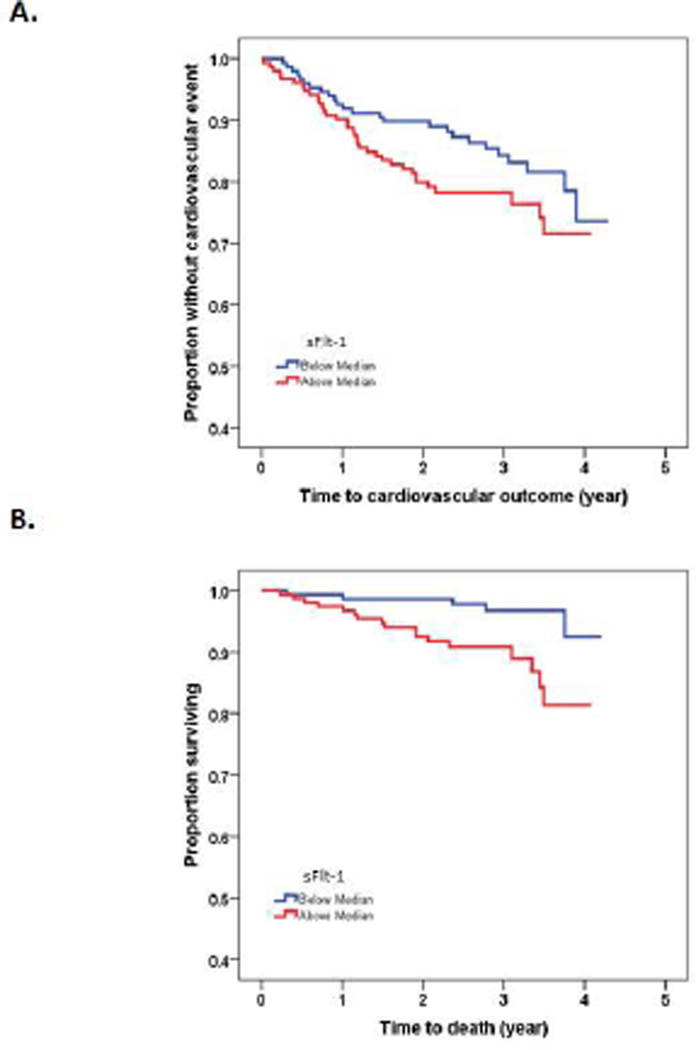
Patients were followed up for a mean of 2.7±0.9 years with maximum follow up time up to 4 years. During the follow up time, 60 patients (19.9%) experienced a cardiovascular event and 22 patients (7.3%) died from cardiac (n=12) and non-cardiac causes (n=10). Kaplan Meir analysis showed that CKD patient with PlGF above median had higher CV event (Fig. 1A, log-rank test p=0.014) and mortality (Fig. 1B, log-rank test p=0.004). Compared with low PlGF, patients with PlGF above median level had higher CV events (12.7% vs. 27.2%, p=0.002) and mortality (2.0% vs. 12.6%, p < 0.001). Patients with sFlt-1 above median had higher mortality rate (Fig. 2B, log-rank test p=0.005), but there was no statically significant difference in CV event in patients with sFlt-1 above median vs. those below median (Fig. 2A, log-rank test p=0.10).
Figure 1.
Kaplan-Meier analysis showing the association of placental growth factor (PlGF) with cardiovascular event (A, log-rank test p=0.014) and mortality (B; log-rank test p=0.004) in 301 patients with CKD
Figure 2.
Kaplan-Meier analysis showing association of soluble fms-like tyrosine kinase-1 (sFlt-1) with cardiovascular events (A, log-rank test p=0.102) and mortality (B, log-rank test p=0.005) in 301 patients with CKD
Unadjusted and multivariate-adjusted Cox proportional hazard ratios of cardiovascular events and mortality are reported in Table 3. The risk for CV event rate was significantly higher among patients with PlGF above median when compared to those with PlGF below median level (HR=1.97, 95% CI: 1.14–3.40, p=0.016), but lost significance after adjusting for sFlt-1, age, sex, BMI, diabetes, smoking, SBP, LDL-C, albuminuria, and eGFR. In order to understand the graded relationship between PIGF level and CV outcome, we repeated the analysis using PIGF as continuous variable and noted a borderline significant association (HR 1.75, 95% CI: 0.98–3.13, p=0.058). (Supplementary table 2) There was no association between sFlt-1 level and CV outcomes.
Table 3.
Unadjusted and multivariate adjusted hazard ratio of cardiovascular events and mortality in patients with CKD stages 2 to 4 with high level of placental endothelial growth factor: above median verses below median (reference group)
|
PlGF above vs. below median |
sFlt-1 above vs. below median |
|||
|---|---|---|---|---|
| Hazard ratio (95% CI) |
p value | Hazard ratio (95% CI) |
p value | |
| CV event | ||||
| Unadjusted | 1.97 (1.14–3.40) | 0.016 | 1.53 (0.92–2.56) | 0.10 |
| Model 1 | 1.94 (1.12–3.36) | 0.018 | 1.50 (0.90–2.51) | 0.12 |
| Model 2 | 1.98 (1.14–3.43) | 0.015 | 1.42 (0.85–2.39) | 0.19 |
| Model 3 | 1.96 (1.10–3.48) | 0.022 | 1.43 (0.84–2.43) | 0.19 |
| Model 4 | 1.002 (0.934–1.375) | 0.95 | 1.34 (0.79–2.28) | 0.28 |
| Mortality | ||||
| Unadjusted | 5.0 (1.47–17.05) | 0.01 | 3.78 (1.39–10.28) | 0.009 |
| Model 1 | 4.82 (1.41–16.48) | 0.012 | 3.62 (1.33–9.82) | 0.012 |
| Model 2 | 5.17 (1.51–17.67) | 0.009 | 3.43 (1.26–9.35) | 0.016 |
| Model 3 | 5.43 (1.56–18.90) | 0.008 | 3.46 (1.25–9.58) | 0.017 |
| Model 4 | 5.22 (1.49–18.33) | 0.01 | 3.41 (1.49–9.51) | 0.019 |
PlGF: placental growth factor; sFlt-1: soluble vascular endothelial growth factor receptor-1; BMI: body mass index; SBP: systolic blood pressure; LDL-C: low density lipoprotein cholesterol; eGFR: estimated glomerular filtration rate
Model 1: PlGF, sFlt-1
Model 2: PlGF, sFlt-1, age, and sex
Model 3: PlGF, sFlt-1, age, sex, BMI, diabetes, smoking, SBP and LDL-C
Model 4: PlGF, sFlt-1, age, sex, BMI, diabetes, smoking, SBP, LDL-C, albuminuria, and eGFR
Higher PlGF was associated with 5 times greater risk of mortality in CKD patients (HR=5.22, 95% CI: 1.49–18.33, p=0.01). This increased death risk was robust to adjustment for sFlt-1 and other risk factors. Similarly, higher sFlt-1 was associated with increased death risk in the fully adjusted model (HR 3.41, 95% CI: 1.49-9.51, p=0.019). The PlGF/sFlt-1 ratio was not associated with CV events or mortality (data not shown). When treated as continuous variables, PlGF remained a significant predictor of mortality (HR 1.136 (95% CI 1.024-1.261, p=0.016), but sFlt-1 did not have a significant association with mortality (HR 1.011 (95% CI 0.998-1.024, p=0.092). (Supplementary table 2)
4. Discussion
In this study of 301 patients with CKD stages 2 to 4, we showed that higher circulating levels of vascular endothelial factors PlGF and sFlt-1 are associated with 5.2 fold and 3.4 fold increased risk for death, respectively. The associations were robust and persisted even after adjusting for a number of known confounding variables. CIMT, a surrogate measure of atherosclerosis was not associated with either of the vascular endothelial factors. Although, PlGF was associated with CV events in the unadjusted model, it did not retain significance in the fully adjusted model.
PLGF (molecular weight 29.7 kDa) is freely filtered into the urine, but sFlt-1 (molecular weight 110-kDa) is not filtered through the glomerular barrier, under physiological conditions. Glomerular epithelial cells and tubular cells exhibit VEGF expression.[14] Injection of sFlt-1 causes hypertension, proteinuria, and glomerular endotheliosis in rats.[15] PlGF and sFlt-1 levels are reported to be increased in patients with impaired renal function.[8, 16] On the other hand, Onoue et al. showed that renal production, as well as the plasma level of sFlt-1, decreased with decline in eGFR.[17] In a study of 114 patients, including 45 CKD patients, 31 hemodialysis patients, and 38 age-matched controls, Zakiyanov et al. found that PlGF was significantly increased in the CKD and hemodialysis patients compared to age-matched healthy controls.[18] Proteinuria appears to be an effect common to all agents targeting the VEGF pathway.[19] In our study, PlGF and sFlt-1 levels were negatively correlated with eGFR and positively correlated with albuminuria.
PlGF is a pro-atherogenic cytokine that induces vascular smooth muscle cell proliferation, monocyte chemotaxis, plaque inflammation, and plaque instability. [20–22] PlGF was found to be upregulated within atherosclerotic lesions in both early and advanced stages.[21, 23] In our study, higher PlGF concentration was correlated with lower HDL-C and higher triglycerides but not LDL-C, and CRP. The role of VEGF and sFlt-1 in the pathogenesis of atherosclerosis remains unclear.[24, 25] In vitro studies show that sFlt-1 binds VEGF, inhibits the binding of VEGF to endothelial cells and prevents cell migration.[26] The Salzburg Atherosclerosis Prevention Program in Subjects at High Individual Risk (SAPHIR) showed that neither VEGF nor sFlt-1 showed an independent association with carotid atherosclerosis.[27] sFlt-1 level was not associated with CIMT in our study population as well. Although there was a trend toward higher CIMT across tertiles of PlGF, it was not statistically significant (Supplemental Fig. 3).
In addition to atherosclerosis and plaque instability, PlGF is shown as a prognostic factor of cardiovascular outcome.[28] In 4-year follow up study of 544 participants enrolled in the placebo arm of the c7E3 Fab Anti Platelet Therapy in Unstable REfractory angina (CAPTURE) trial, elevated plasma levels of PlGF was associated increased risk of myocardial infarction and mortality.[29] Elevated PlGF concentration was associated with higher all-cause and cardiovascular death risk in cohort of 190 type 1 diabetes patients who were followed for 10 years.[16] In a nested case-control study of women who were followed for 14 years, Cassidy et al. found a modest association between PlGF and cardiovascular disease.[30] A recent study by Matsui et al. also showed that increased ratio of PlGF to its receptor, sFlt-1, is associated with increased CV events in CKD patients. [31] We did not find any association between sFlt-1 and CV events. Serum PlGF was associated with CV events in the unadjusted model, but not in the fully adjusted model. It is possible that we were not able to demonstrate significant association between serum PlGF and CV event in our study because of a relatively small number of subjects studied.
Matsumoto et al. also noted that PlGF level is not associated with CV events in patients with stable coronary artery disease.[32] They found PlGF/s-Flt-1 ratio to be an independent predictor of all-cause mortality, but not CV events.[32] We found that elevated circulating levels of PlGF and sFlt-1 are associated with a 5.2 and 3.4 fold increase in risk for death.
The ratio of PlGF to sFLT-1 was not associated with any clinical outcomes in our study population. It is possible that the pathophysiological mechanisms of these angiogenic cytokines extend beyond CVD, since the integrity of the microvasculature and immune response is vital for survival. [33–35] Further studies exploring the possible mechanism for the association between these angiogenic cytokines and mortality are warranted.
Our study should be interpreted in light of the following limitations: (a) our study is relatively small sample size which may explain lack of power to detect a moderate effect size and (b) this is a single site study and therefore, the findings of our study need to be confirmed in other populations.
In summary, in this prospective cohort study of CKD 2 to 4 patients, who were followed for up to 4 years, serum level of PlGF and sFlt-1 at baseline were positively associated with higher mortality risk, but not CV events. If future studies confirm the findings of our study, then medications such as Aflibercept that bind selectively to circulatory PlGF and inhibits binding PlGF to its receptor might have some therapeutic effect.[36] To that end, we suggest additional observational and experimental studies to further examine the diagnostic and prognostic values of PlGF and to elucidate the factors that may alter its concentration in CKD patients.
Supplementary Material
Highlights.
We measure serum levels of PlGF and sFlt-1 in 301 CKD patients.
Primary outcomes were CV events and all-cause mortality.
Carotid-intima media thickness (CIMT) was used as marker of atherosclerosis.
Higher PlGF and sFlt-1 were associated with greater death risk, but not CV events.
Acknowledgments
Funding
This work is supported in part by grants R01 DK073665-01A1, 1U01DK099924-01 and 1U01DK099914-01 from the National Institute of Diabetes and Digestive and Kidney Diseases, and grant UL1TR000075 from the NIH National Center for Advancing Translational Sciences, awarded to Dominic Raj.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
None.
References
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004 Sep 23;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 2.Parfrey PS, Foley RN. The clinical epidemiology of cardiac disease in chronic renal failure. J Am Soc Nephrol. 1999 Jul;10(7):1606–1615. doi: 10.1681/ASN.V1071606. [DOI] [PubMed] [Google Scholar]
- 3.Gupta J, Mitra N, Kanetsky PA, Devaney J, Wing MR, Reilly M, et al. Association between albuminuria, kidney function, and inflammatory biomarker profile. Clin J Am Soc Nephrol. 2012 Sep 27;7:1938–1946. doi: 10.2215/CJN.03500412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raj DS, Shah VO, Rambod M, Kovesdy CP, Kalantar-Zadeh K. Association of Soluble Endotoxin Receptor CD14 and Mortality Among Patients Undergoing Hemodialysis. Am J Kidney Dis. 2009 Aug 19; doi: 10.1053/j.ajkd.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002 Nov 1;20(21):4368–4380. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- 6.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997 Feb;18(1):4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 7.Maglione D, Guerriero V, Viglietto G, Delli-Bovi P, Persico MG. Isolation of a human placenta cDNA coding for a protein related to the vascular permeability factor. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9267–9271. doi: 10.1073/pnas.88.20.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Marco GS, Reuter S, Hillebrand U, Amler S, Konig M, Larger E, et al. The soluble VEGF receptor sFlt1 contributes to endothelial dysfunction in CKD. J Am Soc Nephrol. 2009 Oct;20(10):2235–2245. doi: 10.1681/ASN.2009010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shibuya M. Vascular endothelial growth factor receptor-1 (VEGFR-1/Flt-1): a dual regulator for angiogenesis. Angiogenesis. 2006;9(4):225–230. doi: 10.1007/s10456-006-9055-8. [DOI] [PubMed] [Google Scholar]
- 10.Pilarczyk K, Sattler KJ, Galili O, Versari D, Olson ML, Meyer FB, et al. Placenta growth factor expression in human atherosclerotic carotid plaques is related to plaque destabilization. Atherosclerosis. 2008 Jan;196(1):333–340. doi: 10.1016/j.atherosclerosis.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 11.Hochholzer W, Reichlin T, Stelzig C, Hochholzer K, Meissner J, Breidthardt T, et al. Impact of soluble fms-like tyrosine kinase-1 and placental growth factor serum levels for risk stratification and early diagnosis in patients with suspected acute myocardial infarction. Eur Heart J. 2011 Feb;32(3):326–335. doi: 10.1093/eurheartj/ehq429. [DOI] [PubMed] [Google Scholar]
- 12.Seiler S, Rogacev KS, Roth HJ, Shafein P, Emrich I, Neuhaus S, et al. Associations of FGF-23 and sKlotho with Cardiovascular Outcomes among Patients with CKD Stages 2-4. Clin J Am Soc Nephrol. 2014 Mar 27; doi: 10.2215/CJN.07870713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burnham KP, Anderson DR. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach (2nd ed) 2nd ed. edn. Springer-Verlag; 2002. [Google Scholar]
- 14.Kretzler M, Schroppel B, Merkle M, Huber S, Mundel P, Horster M, et al. Detection of multiple vascular endothelial growth factor splice isoforms in single glomerular podocytes. Kidney Int Suppl. 1998 Sep;67:S159–S161. doi: 10.1046/j.1523-1755.1998.06733.x. [DOI] [PubMed] [Google Scholar]
- 15.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003 Mar;111(5):649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Theilade S, Lajer M, Jorsal A, Tarnow L, Parving HH, Rossing P. Evaluation of placental growth factor and soluble Fms-like tyrosine kinase 1 as predictors of all-cause and cardiovascular mortality in patients with Type 1 diabetes with and without diabetic nephropathy. Diabet Med. 2012 Mar;29(3):337–344. doi: 10.1111/j.1464-5491.2011.03482.x. [DOI] [PubMed] [Google Scholar]
- 17.Onoue K, Uemura S, Takeda Y, Somekawa S, Iwama H, Imagawa K, et al. Reduction of circulating soluble fms-like tyrosine kinase-1 plays a significant role in renal dysfunction-associated aggravation of atherosclerosis. Circulation. 2009 Dec 15;120(24):2470–2477. doi: 10.1161/CIRCULATIONAHA.109.867929. [DOI] [PubMed] [Google Scholar]
- 18.Zakiyanov O, Kalousova M, Zima T, Tesar V. Placental growth factor in patients with decreased renal function. Ren Fail. 2011;33(3):291–297. doi: 10.3109/0886022X.2011.560402. [DOI] [PubMed] [Google Scholar]
- 19.Zhu X, Wu S, Dahut WL, Parikh CR. Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: systematic review and meta-analysis. Am J Kidney Dis. 2007 Feb;49(2):186–193. doi: 10.1053/j.ajkd.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 20.Khurana R, Moons L, Shafi S, Luttun A, Collen D, Martin JF, et al. Placental growth factor promotes atherosclerotic intimal thickening and macrophage accumulation. Circulation. 2005 May 31;111(21):2828–2836. doi: 10.1161/CIRCULATIONAHA.104.495887. [DOI] [PubMed] [Google Scholar]
- 21.Roncal C, Buysschaert I, Gerdes N, Georgiadou M, Ovchinnikova O, Fischer C, et al. Short-term delivery of anti-PlGF antibody delays progression of atherosclerotic plaques to vulnerable lesions. Cardiovasc Res. 2010 Apr 1;86(1):29–36. doi: 10.1093/cvr/cvp380. [DOI] [PubMed] [Google Scholar]
- 22.Markovic M, Ignjatovic S, Dajak M, Majkic-Singh N. Placental growth factor as short-term predicting biomarker in acute coronary syndrome patients with non-ST elevation myocardial infarction. South Med J. 2010 Oct;103(10):982–987. doi: 10.1097/SMJ.0b013e3181eda4ef. [DOI] [PubMed] [Google Scholar]
- 23.Koenig W, Khuseyinova N. Biomarkers of atherosclerotic plaque instability and rupture. Arterioscler Thromb Vasc Biol. 2007 Jan;27(1):15–26. doi: 10.1161/01.ATV.0000251503.35795.4f. [DOI] [PubMed] [Google Scholar]
- 24.Luttun A, Tjwa M, Carmeliet P. Placental growth factor (PlGF) and its receptor Flt-1 (VEGFR-1): novel therapeutic targets for angiogenic disorders. Ann N Y Acad Sci. 2002 Dec;979:80–93. doi: 10.1111/j.1749-6632.2002.tb04870.x. [DOI] [PubMed] [Google Scholar]
- 25.Celletti FL, Waugh JM, Amabile PG, Brendolan A, Hilfiker PR, Dake MD. Vascular endothelial growth factor enhances atherosclerotic plaque progression. Nat Med. 2001 Apr;7(4):425–429. doi: 10.1038/86490. [DOI] [PubMed] [Google Scholar]
- 26.Hornig C, Barleon B, Ahmad S, Vuorela P, Ahmed A, Weich HA. Release and complex formation of soluble VEGFR-1 from endothelial cells and biological fluids. Lab Invest. 2000 Apr;80(4):443–454. doi: 10.1038/labinvest.3780050. [DOI] [PubMed] [Google Scholar]
- 27.Sandhofer A, Tatarczyk T, Kirchmair R, Iglseder B, Paulweber B, Patsch JR, et al. Are plasma VEGF and its soluble receptor sFlt-1 atherogenic risk factors? Cross-sectional data from the SAPHIR study. Atherosclerosis. 2009 Sep;206(1):265–269. doi: 10.1016/j.atherosclerosis.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 28.Bui AH, Bonaca MP, Sabatine MS, Ray KK, Rifai N, Cannon CP, et al. Elevated concentration of placental growth factor (PlGF) and long term risk in patients with acute coronary syndrome in the PROVE IT-TIMI 22 trial. J Thromb Thrombolysis. 2012 Aug;34(2):222–228. doi: 10.1007/s11239-012-0704-z. [DOI] [PubMed] [Google Scholar]
- 29.Lenderink T, Heeschen C, Fichtlscherer S, Dimmeler S, Hamm CW, Zeiher AM, et al. Elevated placental growth factor levels are associated with adverse outcomes at four-year follow-up in patients with acute coronary syndromes. J Am Coll Cardiol. 2006 Jan 17;47(2):307–311. doi: 10.1016/j.jacc.2005.08.063. [DOI] [PubMed] [Google Scholar]
- 30.Cassidy A, Chiuve SE, Manson JE, Rexrode KM, Girman CJ, Rimm EB. Potential role for plasma placental growth factor in predicting coronary heart disease risk in women. Arterioscler Thromb Vasc Biol. 2009 Jan;29(1):134–139. doi: 10.1161/ATVBAHA.108.171066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsui M, Takeda Y, Uemura S, Matsumoto T, Seno A, Onoue K, et al. Suppressed soluble Fms-like tyrosine kinase-1 production aggravates atherosclerosis in chronic kidney disease. Kidney Int. 2014 Feb;85(2):393–403. doi: 10.1038/ki.2013.339. [DOI] [PubMed] [Google Scholar]
- 32.Matsumoto T, Uemura S, Takeda Y, Matsui M, Okada S, Nishida T, et al. An elevated ratio of placental growth factor to soluble fms-like tyrosine kinase-1 predicts adverse outcomes in patients with stable coronary artery disease. Intern Med. 2013;52(10):1019–1027. doi: 10.2169/internalmedicine.52.9073. [DOI] [PubMed] [Google Scholar]
- 33.Bobic S, Seys S, De VV, Callebaut I, Hox V, Dooms C, et al. Placental growth factor contributes to bronchial neutrophilic inflammation and edema in allergic asthma. Am J Respir Cell Mol Biol. 2012 Jun;46(6):781–789. doi: 10.1165/rcmb.2011-0152OC. [DOI] [PubMed] [Google Scholar]
- 34.Yano K, Okada Y, Beldi G, Shih SC, Bodyak N, Okada H, et al. Elevated levels of placental growth factor represent an adaptive host response in sepsis. J Exp Med. 2008 Oct 27;205(11):2623–2631. doi: 10.1084/jem.20080398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vanheule E, Fan YD, Van HJ, Meester D, Olievier K, Praet M, et al. Expression of placental growth factor in regenerating livers after partial hepatectomy in the rat. Eur J Gastroenterol Hepatol. 2011 Jan;23(1):66–75. doi: 10.1097/MEG.0b013e328341ef35. [DOI] [PubMed] [Google Scholar]
- 36.Papadopoulos N, Martin J, Ruan Q, Rafique A, Rosconi MP, Shi E, et al. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis. 2012 Jun;15(2):171–185. doi: 10.1007/s10456-011-9249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.