Abstract
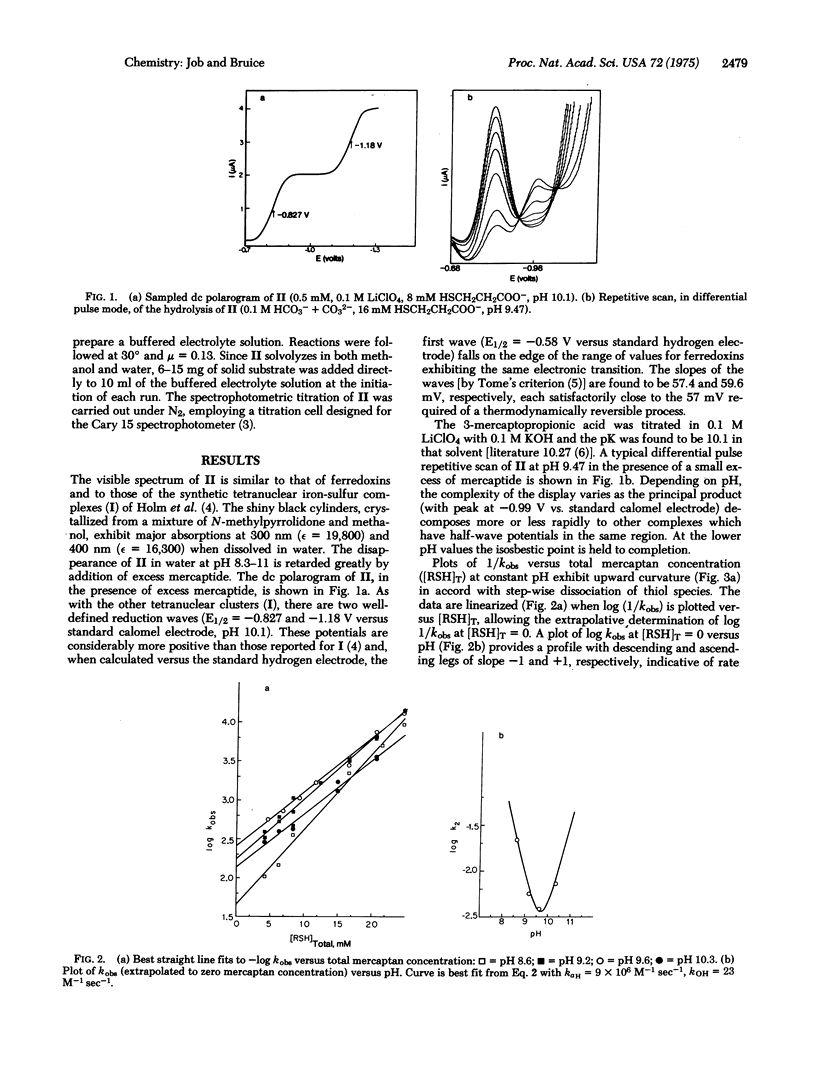
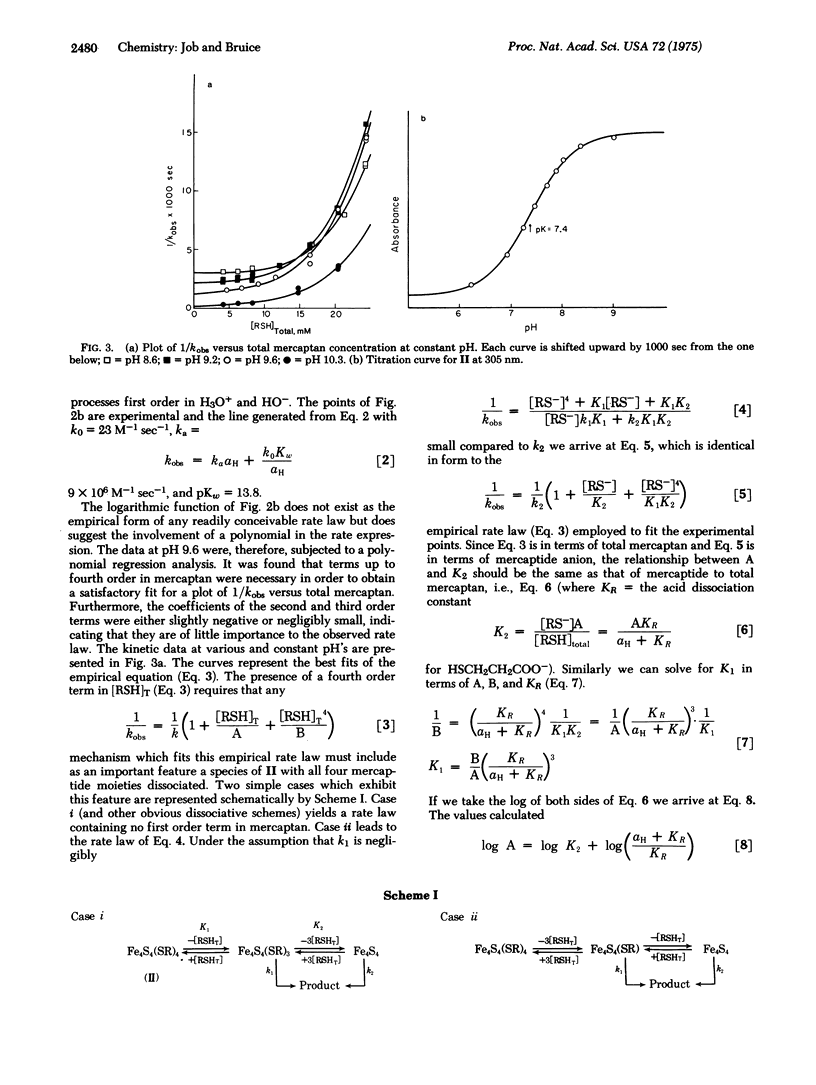
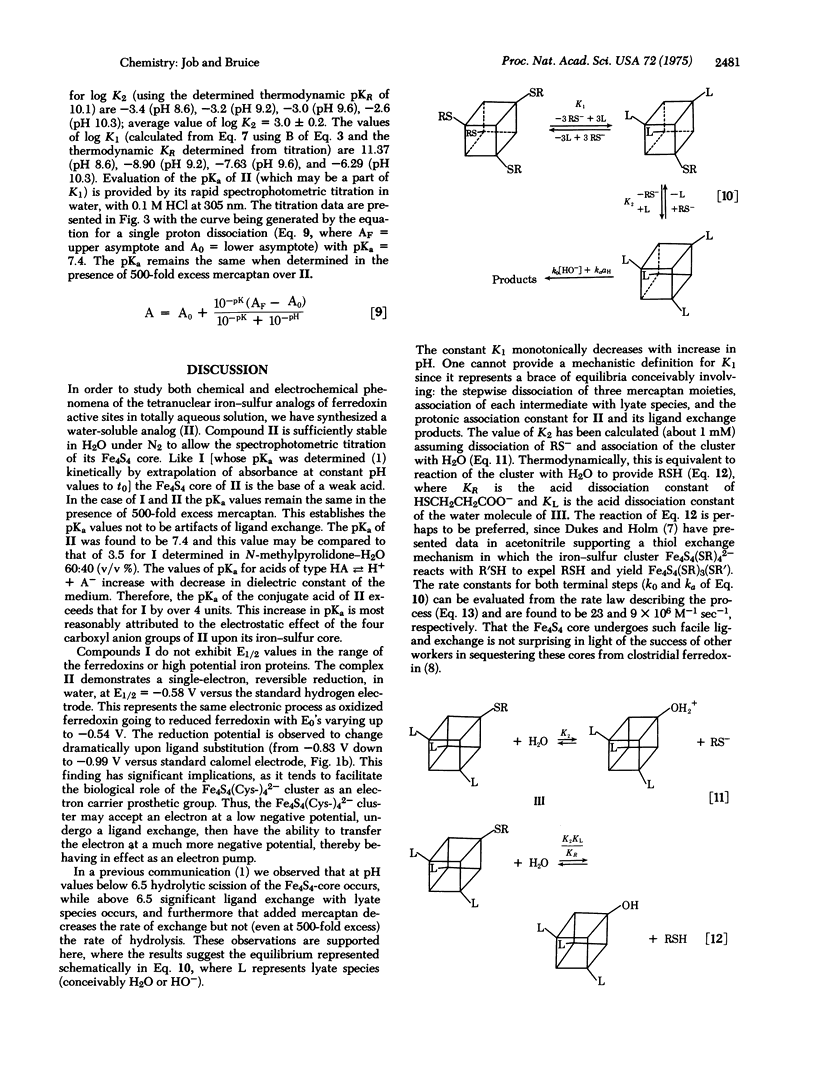
The water-soluble tetranuclear iron-sulfur cluster ion Fe4S4(SCH2CH2CO2-)46- (II) has been prepared. The stability of II in water is sufficient to allow the spectrotitrimetric determination of the pKa of its Fe4S4 core as 7.4. In our hands the one-electron reductions of compounds I [Fe4S4(SR)42-, R = alkyl or aryl,] are thermodynamically irreversible with associated E1/2 values greater than those for one-electron reduction of ferredoxins. In contrast, the one-electron reduction of II is thermodynamically reversible and the associated potential (-0.58 V versus hydrogen electrode) approaches closely that of the ferredoxins. The kinetics for ligand exchange of II as a function of pH and thiol concentration are in accord with four reversible mercaptan/lyate species exchange reactions followed by product formation via specific acid and base catalysis. Preliminary experiments indicate the nucleophilic order towards II to be Cl- [unk] Br- < HO- < CN-.
Keywords: ferredoxin, nucleophilicity
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Averill B. A., Herskovitz T., Holm R. H., Ibers J. A. Synthetic analogs of the active sites of iron-sulfur proteins. II. Synthesis and structure of the tetra(mercapto-m 3 -sulfido-iron) clusters, (Fe 4 S 4 (SR) 4 ) 2- . J Am Chem Soc. 1973 May 30;95(11):3523–3534. doi: 10.1021/ja00792a013. [DOI] [PubMed] [Google Scholar]
- Bruice T. C., Maley J. R. Equilibrium titration and pH-stat cell for a Cary 15 spectrophotometer. Anal Biochem. 1970 Mar;34:275–278. doi: 10.1016/0003-2697(70)90105-3. [DOI] [PubMed] [Google Scholar]
- Bruice T. C., Maskiewicz R., Job R. The Acid-Base Properties, Hydrolytic Mechanism, and Susceptibility to O(2) Oxidation of Fe(4)S(4)(SR)(4) Clusters. Proc Natl Acad Sci U S A. 1975 Jan;72(1):231–234. doi: 10.1073/pnas.72.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePamphilis B. V., Averill B. A., Herskovitz T., Que L., Jr, Holm R. H. Synthetic analogs of the active sites of iron-sulfur proteins. VI. Spectral and redox characteristics of the tetranuclear clusters (Fe4S4(SR)4).2-. J Am Chem Soc. 1974 Jun 26;96(13):4159–4167. doi: 10.1021/ja00820a017. [DOI] [PubMed] [Google Scholar]
- Dukes G. R., Holm R. H. Synthetic analogs of the active sites of iron-sulfur proteins; X. Kinetics and mechanism of the ligand substitution reactions of arylthiols with the tetranuclear clusters [Fe4S4(SR)4]2 minus;. J Am Chem Soc. 1975 Feb 5;97(3):528–533. doi: 10.1021/ja00836a011. [DOI] [PubMed] [Google Scholar]
- Que L., Jr, Holm R. H., Mortenson L. E. Letter: Extrusion of Fe2S2 and Fe4S4 cores from the active sites of ferredoxin proteins. J Am Chem Soc. 1975 Jan 22;97(2):463–464. doi: 10.1021/ja00835a064. [DOI] [PubMed] [Google Scholar]


