Abstract
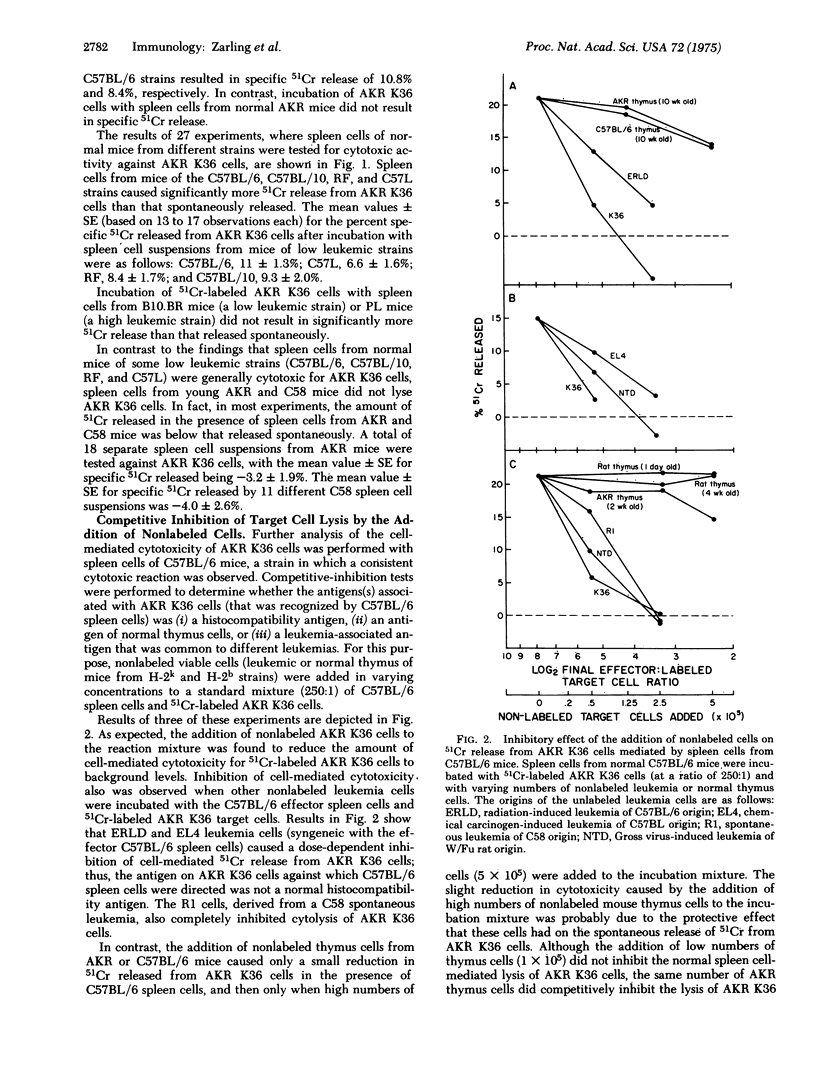
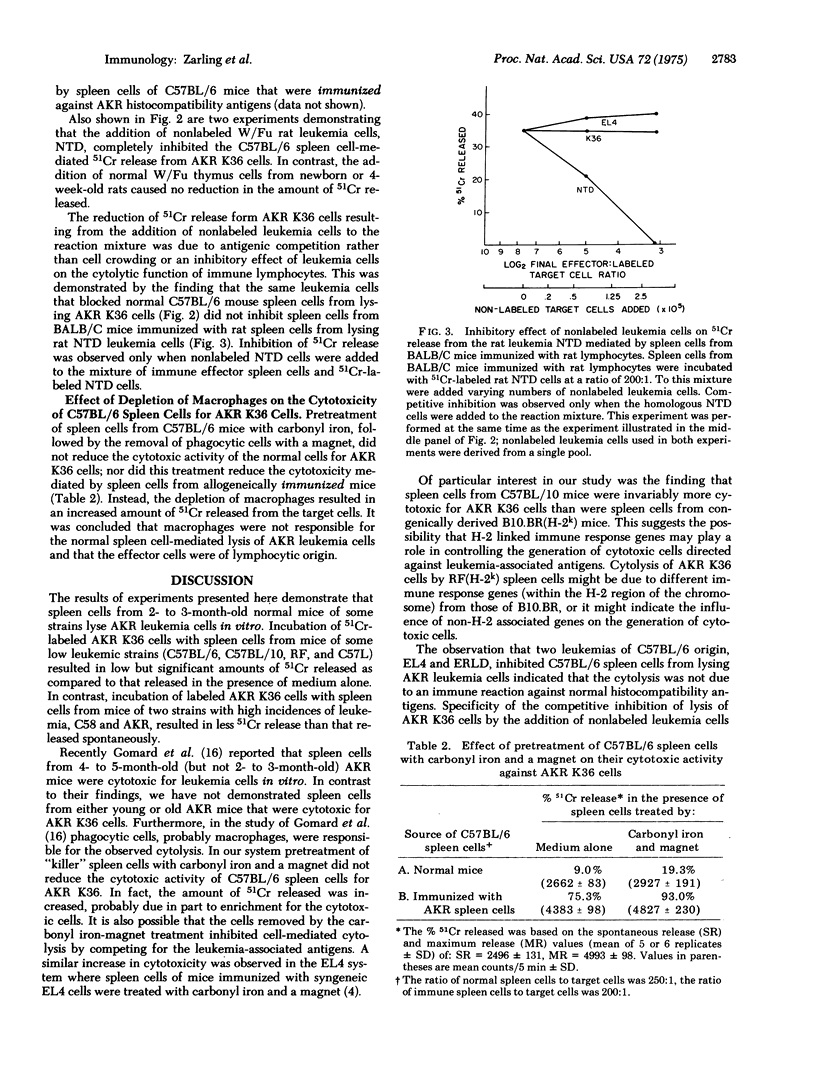
Spleen cells from 2- to 3-month-old normal mice of some strains having a low incidence of spontaneous leukemia were found to lyse cells of the spontaneous AKR leukemia K36 in the 51Cr release assay. Incubation of 51Cr-labeled ADR K36 cells with spleen cells from normal C57BL/6, C57L, C57BL/10, and RF mice resulted in the release of significantly more 51Cr than that released in the presence of medium alone. In contrast, 51Cr released from AKR K36 cells after incubation with spleen cells from mice of the high leukemic strains AKR and C58 was less than that released spontaneously. The results of competitive inhibition tests when C57BL/6 spleen cells were incubated simultaneously with 51Cr-labeled AKR K36 target cells and varying numbers of nonlabeled cells demonstrated that the cytotoxic activity of normal C57BL/6 spleen cells was directed against an antigen(s) associated with several leukemias, but that was undetectable on normal thymocytes. Pretreatment of C57BL/6 spleen cells with carbonyl iron and a magnet, which removed phagocytic macrophages, did not decrease the cytotoxic acitivity for AKR K36 cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Stephenson J. R. Widespread natural occurrence of high titers of neutralizing antibodies to a specific class of endogenous mouse type-C virus. Proc Natl Acad Sci U S A. 1974 May;71(5):1957–1961. doi: 10.1073/pnas.71.5.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki T., Boyse E. A., Old L. J. Occurrence of natural antibody to the G (gross) leukemia antigen in mice. Cancer Res. 1966 Jul;26(7):1415–1419. [PubMed] [Google Scholar]
- Aoki T., Huebner R. J., Chang K. S., Sturm M. M., Liu M. Diversity of envelope antigens on murine type-C RNA viruses. J Natl Cancer Inst. 1974 Apr;52(4):1189–1197. doi: 10.1093/jnci/52.4.1189. [DOI] [PubMed] [Google Scholar]
- Cerottini J. C., Nordin A. A., Brunner K. T. Specific in vitro cytotoxicity of thymus-derived lymphocytes sensitized to alloantigens. Nature. 1970 Dec 26;228(5278):1308–1309. doi: 10.1038/2281308a0. [DOI] [PubMed] [Google Scholar]
- Colnaghi M. I., Della Porta G. Evidence for virus-related and unrelated antigens on murine lymphomas induced by chemical carcinogens. J Natl Cancer Inst. 1973 Jan;50(1):173–180. doi: 10.1093/jnci/50.1.173. [DOI] [PubMed] [Google Scholar]
- GORER P. A. Studies in antibody response of mice to tumour inoculation. Br J Cancer. 1950 Dec;4(4):372–379. doi: 10.1038/bjc.1950.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golstein P., Schirrmacher V., Rubin B., Wigzell H. Cytotoxic immune cells with specificity for defined soluble antigens. II. Chasing the killing cells. Cell Immunol. 1973 Nov;9(2):211–225. doi: 10.1016/0008-8749(73)90072-5. [DOI] [PubMed] [Google Scholar]
- Gomard E., Leclerc J. C., Levy J. P. Spontaneous antilymphoma reaction of preleukaemic AKR mice is a non-T-cell killing. Nature. 1974 Aug 23;250(5468):671–673. doi: 10.1038/250671a0. [DOI] [PubMed] [Google Scholar]
- Herberman R. B. Cell-mediated immunity to tumor cells. Adv Cancer Res. 1974;19(0):207–263. doi: 10.1016/s0065-230x(08)60055-x. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Nunn M. E., Lavrin D. H., Asofsky R. Effect of antibody to theta antigen on cell-mediated immunity induced in syngeneic mice by murine sarcoma virus. J Natl Cancer Inst. 1973 Nov;51(5):1509–1512. doi: 10.1093/jnci/51.5.1509. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Yurconic M., Jr, Hanna M. G., Jr Autogenous immunity to endogenous RNA tumor virus. Radioimmune precipitation assay of mouse serum antibody levels. J Exp Med. 1973 Jul 1;138(1):194–208. doi: 10.1084/jem.138.1.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiessling R., Klein E., Pross H., Wigzell H. "Natural" killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur J Immunol. 1975 Feb;5(2):117–121. doi: 10.1002/eji.1830050209. [DOI] [PubMed] [Google Scholar]
- Kiessling R., Klein E., Wigzell H. "Natural" killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975 Feb;5(2):112–117. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- Leclerc J. C., Gomard E., Plata F., Levy J. P. Cell-mediated immune reaction against tumors induced by oncornaviruses. II. Nature of the effector cells in tumor-cell cytolysis. Int J Cancer. 1973 Mar 15;11(2):426–432. doi: 10.1002/ijc.2910110220. [DOI] [PubMed] [Google Scholar]
- Nowinski R. C., Kaehler S. L. Antibody to leukemia virus: widespread occurrence in inbred mice. Science. 1974 Sep 6;185(4154):869–871. doi: 10.1126/science.185.4154.869. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Aoki T., Dixon F. J. The antibody response of mice to murine leukemia virus in spontaneous infection: absence of classical immunologic tolerance (AKR mice-complement-fixing antibodies-lymphocytic choriomeningitis virus-immunofluorescence-glomerular deposits of antigen-antibody complexes). Proc Natl Acad Sci U S A. 1972 Jan;69(1):134–138. doi: 10.1073/pnas.69.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz de Landazuri M., Herberman R. B. In vitro activation of cellular immune response to Gross virus-induced lymphoma. J Exp Med. 1972 Nov 1;136(5):969–983. doi: 10.1084/jem.136.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shellam G. R. Studies on a gross-virus-induced lymphoma in the rat. I. The cell-mediated immune response. Int J Cancer. 1974 Jul 15;14(1):65–82. doi: 10.1002/ijc.2910140109. [DOI] [PubMed] [Google Scholar]
- Vasudevan D. M., Brunner K. T., Cerottini J. C. Detection of cytotoxic T lymphocytes in the EL4 mouse leukemia system: increased activity of immune spleen and peritoneal cells following preincubation and cell fractionation procedures. Int J Cancer. 1974 Sep 15;14(3):301–313. doi: 10.1002/ijc.2910140303. [DOI] [PubMed] [Google Scholar]


