Abstract
Horizontal lipid bilayer membranes were used as a model system to study lymphocyte-mediated killing of target cells. Dinitrophenylated lipid bilayers can physically support dozens of lymphocytes for periods of over one hour without breakage or increasing the electrical conductance of the membrane. However, in the presence of antibody against Dnp, human lymphocytes rapidly induced increases in membrane conductance of several orders of magnitude without membrane breakage. Such ionic permeability increases occurred only when the membrane voluage was positive on the lymphocyte side, as would be the case with a target cell membrane. The lymphocyte and antibody dependence of this conductance increase parallels that observed for lymphocyte killing of antibody-coated target cells. The results are interpreted as evidence that the primary event in lymphocyte killing of antibody-coated target cells is the creation of ion-conducting channels in the target membrane.
Full text
PDF

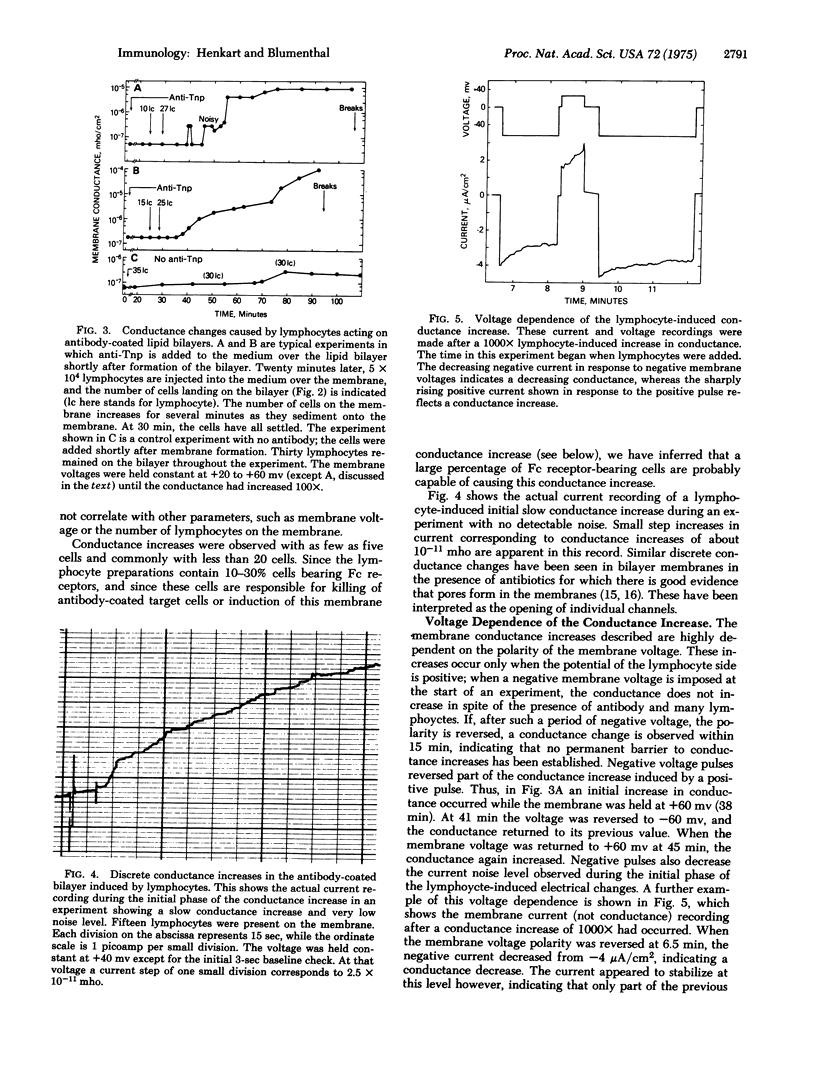

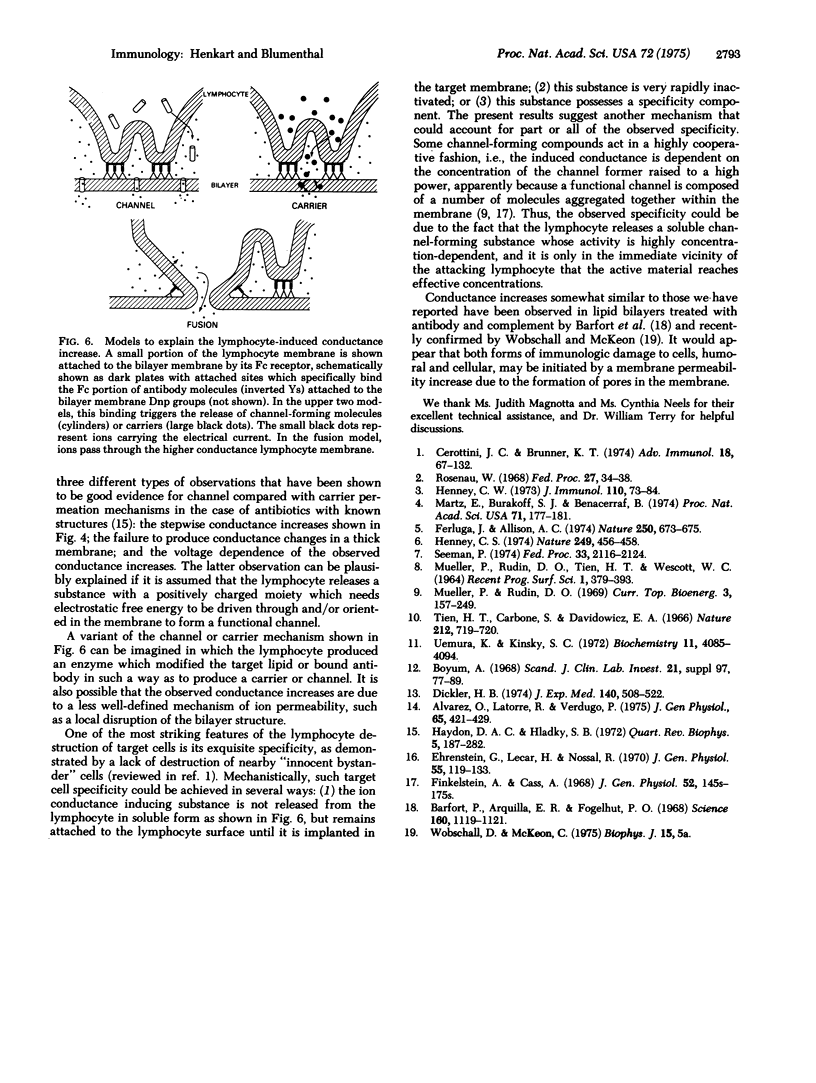
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez O., Latorre R., Verdugo P. Kinetic characteristics of the excitability-inducing material channel in oxidized cholesterol and brain lipid bilayer membranes. J Gen Physiol. 1975 Apr;65(4):421–439. doi: 10.1085/jgp.65.4.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barfort P., Arquilla E. R., Vogelhut P. O. Resistance changes in lipid bilayers: immunological applications. Science. 1968 Jun 7;160(3832):1119–1121. doi: 10.1126/science.160.3832.1119. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cerottini J. C., Brunner K. T. Cell-mediated cytotoxicity, allograft rejection, and tumor immunity. Adv Immunol. 1974;18:67–132. doi: 10.1016/s0065-2776(08)60308-9. [DOI] [PubMed] [Google Scholar]
- Dickler H. B. Studies of the human lymphocyte receptor for heat-aggregated or antigen-complexed immunoglobulin. J Exp Med. 1974 Aug 1;140(2):508–522. doi: 10.1084/jem.140.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenstein G., Lecar H., Nossal R. The nature of the negative resistance in bimolecular lipid membranes containing excitability-inducing material. J Gen Physiol. 1970 Jan;55(1):119–133. doi: 10.1085/jgp.55.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferluga J., Allison A. C. Observations on the mechanism by which T-lymphocytes exert cytotoxic effects. Nature. 1974 Aug 23;250(5468):673–675. doi: 10.1038/250673a0. [DOI] [PubMed] [Google Scholar]
- Haydon D. A., Hladky S. B. Ion transport across thin lipid membranes: a critical discussion of mechanisms in selected systems. Q Rev Biophys. 1972 May;5(2):187–282. doi: 10.1017/s0033583500000883. [DOI] [PubMed] [Google Scholar]
- Henney C. S. Estimation of the size of a T-cell-induced lytic lesion. Nature. 1974 May 31;249(456):456–458. doi: 10.1038/249456a0. [DOI] [PubMed] [Google Scholar]
- Henney C. S. Studies on the mechanism of lymphocyte-mediated cytolysis. II. The use of various target cell markers to study cytolytic events. J Immunol. 1973 Jan;110(1):73–84. [PubMed] [Google Scholar]
- Martz E., Burakoff S. J., Benacerraf B. Interruption of the sequential release of small and large molecules from tumor cells by low temperature during cytolysis mediated by immune T-cells or complement. Proc Natl Acad Sci U S A. 1974 Jan;71(1):177–181. doi: 10.1073/pnas.71.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenau W. Target cell destruction. Fed Proc. 1968 Jan-Feb;27(1):34–38. [PubMed] [Google Scholar]
- Seeman P. Ultrastructure of membrane lesions in immune lysis, osmotic lysis and drug-induced lysis. Fed Proc. 1974 Oct;33(10):2116–2124. [PubMed] [Google Scholar]
- Uemura K., Kinsky S. C. Active vs. passive sensitization of liposomes toward antibody and complement by dinitrophenylated derivatives of phosphatidylethanolamine. Biochemistry. 1972 Oct 24;11(22):4085–4094. doi: 10.1021/bi00772a010. [DOI] [PubMed] [Google Scholar]



