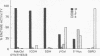Abstract
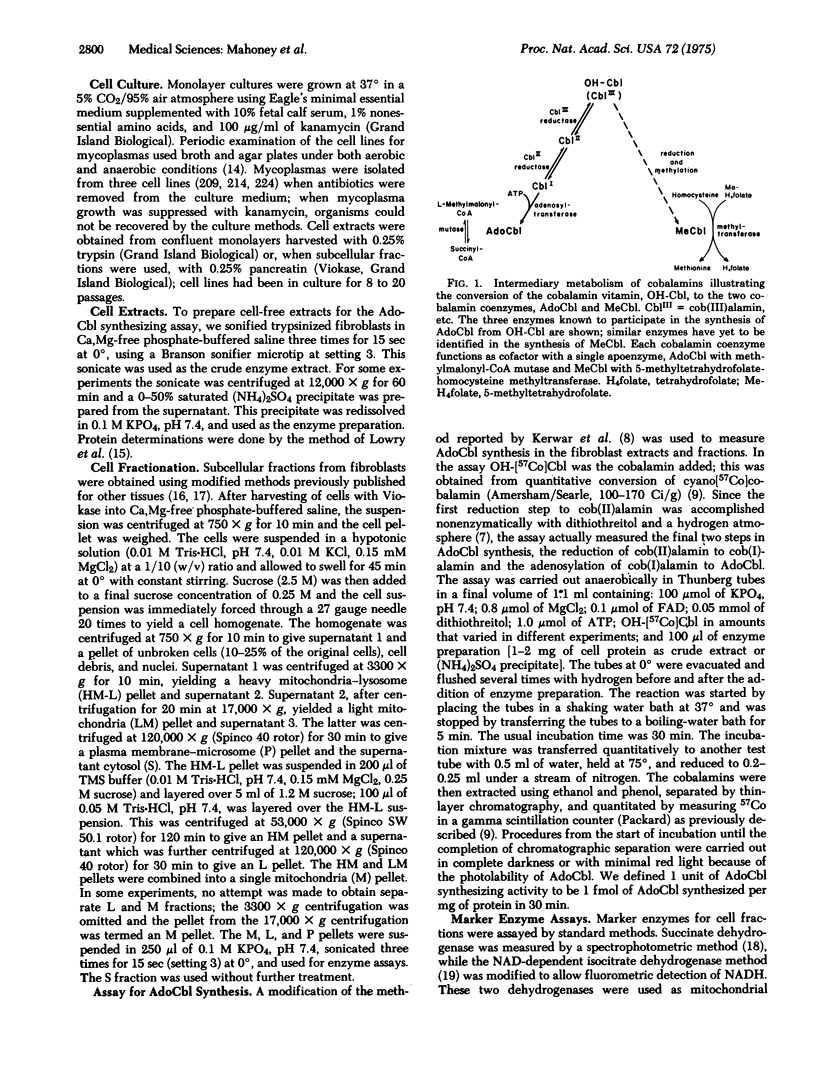
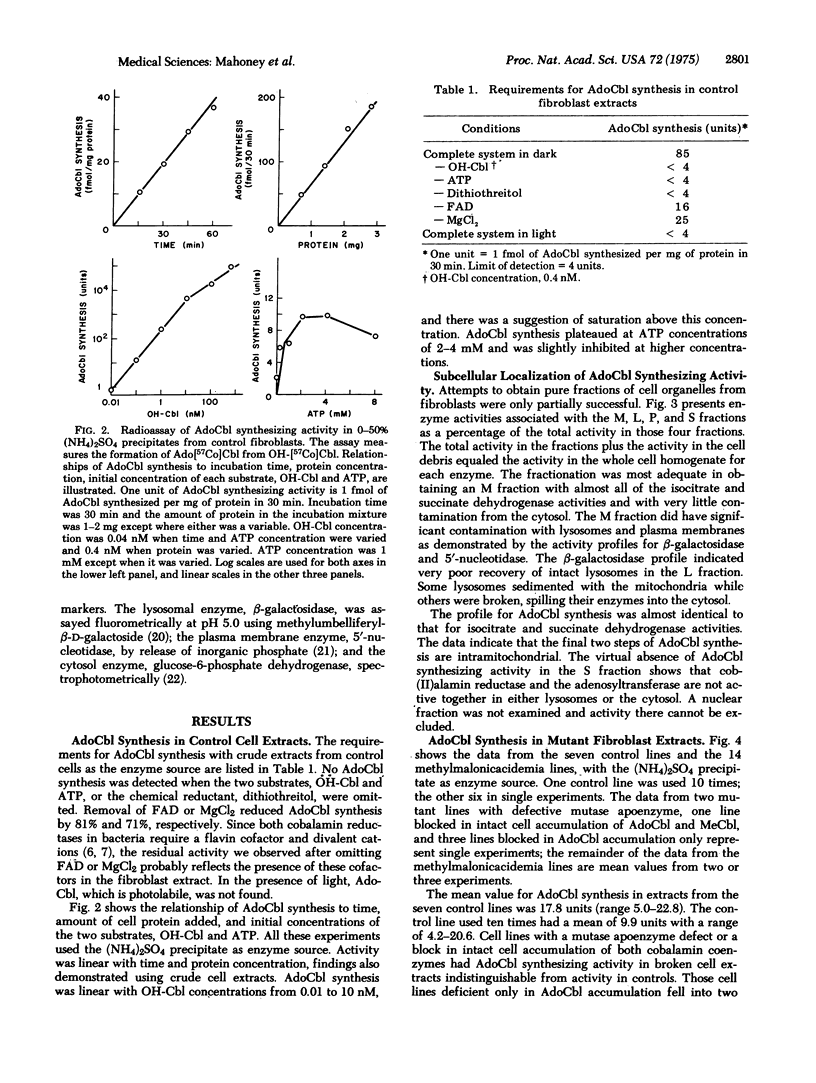
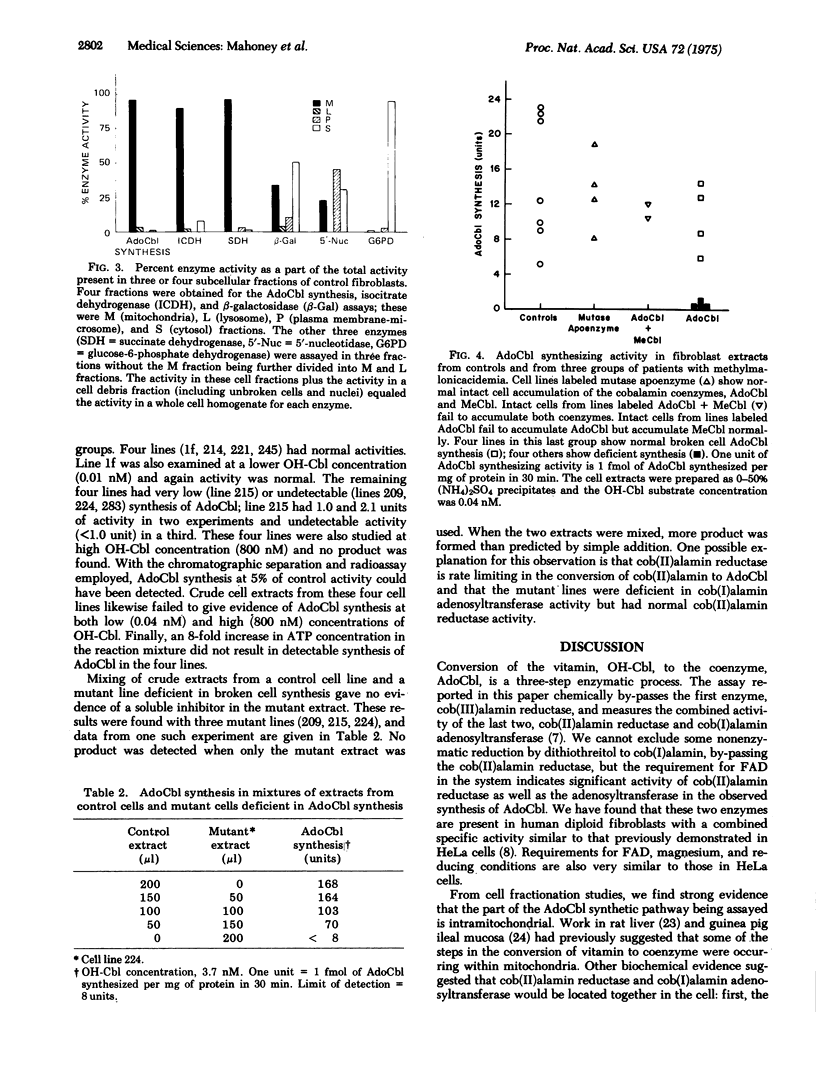
We measured the synthesis of 5'-deoxyadenosylcobalamin (AdoCbl) in fibroblast extracts from patients with inherited methylmalonicacidemia due to deficient activity of the cobalamin-dependent holoenzyme, methylmalonyl-CoA mutase (EC 5.4.99.2). Previous studies with intact fibroblasts from patients whose holoenzyme deficiency was secondary to abnormal cobalamin metabolism had defined two phenotypes, one in which whole cells failed to accumulate AdoCbl and a second in which they failed to accumulate both AdoCbl and the second cobalamin coenzyme, methylcobalamin. With a broken cell assay of AdoCbl synthesis in cell extracts and the cell lines are named cbl A mutants; the other class shows severe deficiency of AdoCbl synthesis and the cell lines are named cbl B mutants. We define cbl C mutants as those in which both AdoCbl and methylcobalamin fail to accumulate in intact cells. The assay for AdoCbl synthesis is thought to measure two enzymatic activities, cob(II)alamin reductase (EC 1.6.99.9) and cob(I)alamin adenosyltransferase (EC 2.5.1.17). Subcellular fractionation studies place this combined activity in mitochondria.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dillon M. J., England J. M., Gompertz D., Goodey P. A., Grant D. B., Hussein H. A., Linnell J. C., Matthews D. M., Mudd S. H., Newns G. H. Mental retardation, megaloblastic anaemia, methylmalonic aciduria and abnormal homocysteine metabolism due to an error in vitamin B12 metabolism. Clin Sci Mol Med. 1974 Jul;47(1):43–61. doi: 10.1042/cs0470043. [DOI] [PubMed] [Google Scholar]
- Goldstone A., Koenig H., Nayyar R., Hughes C., Lu C. Y. Isolation and characterization of a rough microsomal fraction from rat kidney that is enriched in lysosomal enzymes. Biochem J. 1973 Feb;132(2):259–266. doi: 10.1042/bj1320259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang E. S., Snodgrass P. J., Gerald P. S. Methylmalonyl coenzyme A racemase defect: another cause of methylmalonic aciduria. Pediatr Res. 1972 Dec;6(12):875–879. doi: 10.1203/00006450-197212000-00004. [DOI] [PubMed] [Google Scholar]
- Kaye C. I., Morrow G., 3rd, Nadler H. L. In vitro "responsive" methylmalonic acidemia: a new variant. J Pediatr. 1974 Jul;85(1):55–59. doi: 10.1016/s0022-3476(74)80285-4. [DOI] [PubMed] [Google Scholar]
- Kerwar S. S., Spears C., McAuslan B., Weissbach H. Studies on vitamin B12 metabolism in HeLa cells. Arch Biochem Biophys. 1971 Jan;142(1):231–237. doi: 10.1016/0003-9861(71)90279-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Loverde A. W., Lehrer G. M. Subcellular distribution of isocitrate dehydrogenases in neonatal and adult mouse brain. J Neurochem. 1973 Feb;20(2):441–448. doi: 10.1111/j.1471-4159.1973.tb12143.x. [DOI] [PubMed] [Google Scholar]
- Mahoney M. J., Rosenberg L. E., Mudd S. H., Uhlendorf B. W. Defective metabolism of vitamin B 12 in fibroblasts from children with methylmalonicaciduria. Biochem Biophys Res Commun. 1971 Jul 16;44(2):375–381. doi: 10.1016/0006-291x(71)90610-3. [DOI] [PubMed] [Google Scholar]
- Mahoney M. J., Rosenberg L. E. Synthesis of cobalamin coenzymes by human cells in tissue culture. J Lab Clin Med. 1971 Aug;78(2):302–308. [PubMed] [Google Scholar]
- McGarrity G. J., Coriell L. L. Detection of anaerobic mycoplasmas in cell cultures. In Vitro. 1973 Jul-Aug;9(1):17–18. doi: 10.1007/BF02615983. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Hawthorne J. N. The site of diphosphoinositide synthesis in rat liver. Biochem Biophys Res Commun. 1965 Nov 22;21(4):333–338. doi: 10.1016/0006-291x(65)90198-1. [DOI] [PubMed] [Google Scholar]
- Morrow G., 3rd, Barness L. A., Cardinale G. J., Abeles R. H., Flaks J. G. Congenital methylmalonic acidemia: enzymatic evidence for two forms of the disease. Proc Natl Acad Sci U S A. 1969 May;63(1):191–197. doi: 10.1073/pnas.63.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow G., 3rd, Brodie J. D., Strimpler A., Barness L. A. Subcellular distribution of methylmalonyl CoA carbonylmutase in human liver extracts. Biochem Med. 1973 Oct;8(2):240–248. doi: 10.1016/0006-2944(73)90028-8. [DOI] [PubMed] [Google Scholar]
- Mudd S. H., Uhlendorf B. W., Hinds K. R. Deranged B 12 metabolism: studies of fibroblasts grown in tissue culture. Biochem Med. 1970 Nov;4(3):215–239. doi: 10.1016/0006-2944(70)90050-5. [DOI] [PubMed] [Google Scholar]
- Newmark P., Newman G. E., O'Brien J. R. Vitamin B12 in the rat kidney. Evidence for an association with lysosomes. Arch Biochem Biophys. 1970 Nov;141(1):121–130. doi: 10.1016/0003-9861(70)90114-1. [DOI] [PubMed] [Google Scholar]
- Oberholzer V. G., Levin B., Burgess E. A., Young W. F. Methylmalonic aciduria. An inborn error of metabolism leading to chronic metabolic acidosis. Arch Dis Child. 1967 Oct;42(225):492–504. doi: 10.1136/adc.42.225.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters T. J., Hoffbrand A. V. Absorption of vitamin B 12 by the guinea-pig. I. Subcellular localization of vitamin B 12 in the ileal enterocyte during absorption. Br J Haematol. 1970 Sep;19(3):369–382. doi: 10.1111/j.1365-2141.1970.tb01634.x. [DOI] [PubMed] [Google Scholar]
- Robinson D., Price R. G., Dance N. Separation and properties of beta-galactosidase, beta-glucosidase, beta-glucuronidase and N-acetyl-beta-glucosaminidase from rat kidney. Biochem J. 1967 Feb;102(2):525–532. doi: 10.1042/bj1020525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg L. E., Lilljeqvist A. C., Hsia Y. E., Rosenbloom F. M. Vitamin B12 dependent methylmalonicaciduria: defective B12 metabolism in cultured fibroblasts. Biochem Biophys Res Commun. 1969 Nov 6;37(4):607–614. doi: 10.1016/0006-291x(69)90853-5. [DOI] [PubMed] [Google Scholar]
- Vitols E., Walker G. A., Huennekens F. M. Enzymatic conversion of vitamin B-12s to a cobamide coenzyme, alpha-(5,6-dimethylbenzimidazolyl)deoxyadenosylcobamide (adenosyl-B-12). J Biol Chem. 1966 Apr 10;241(7):1455–1461. [PubMed] [Google Scholar]
- Walker G. A., Murphy S., Huennekens F. M. Enzymatic conversion of vitamin B 12a to adenosyl-B 12: evidence for the existence of two separate reducing systems. Arch Biochem Biophys. 1969 Oct;134(1):95–102. doi: 10.1016/0003-9861(69)90255-0. [DOI] [PubMed] [Google Scholar]
- Wang F. K., Koch J., Stokstad E. L. Folate coenzyme pattern, folate linked enzymes and methionine biosynthesis in rat liver mitochondria. Biochem Z. 1967 Jan 27;346(5):458–466. [PubMed] [Google Scholar]
- Yagil G., Feldman M. The stability of some enzymes in cultured cells. Exp Cell Res. 1969 Jan;54(1):29–36. doi: 10.1016/0014-4827(69)90288-2. [DOI] [PubMed] [Google Scholar]