Abstract
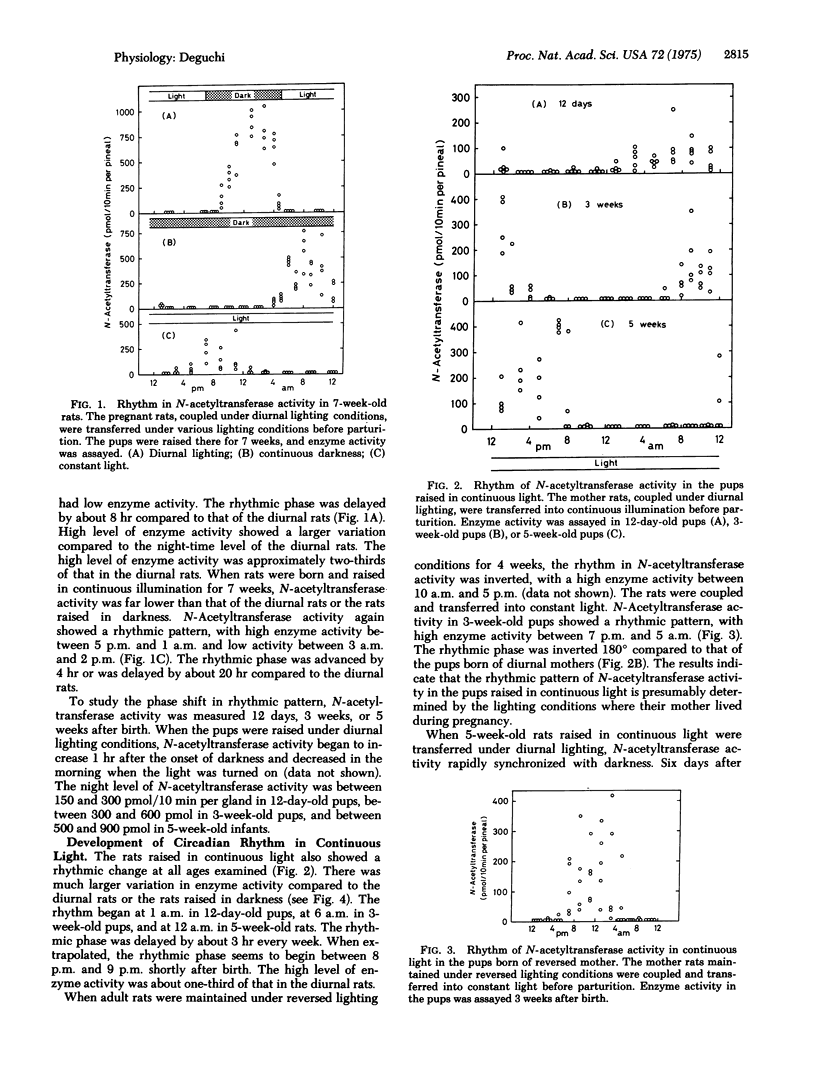
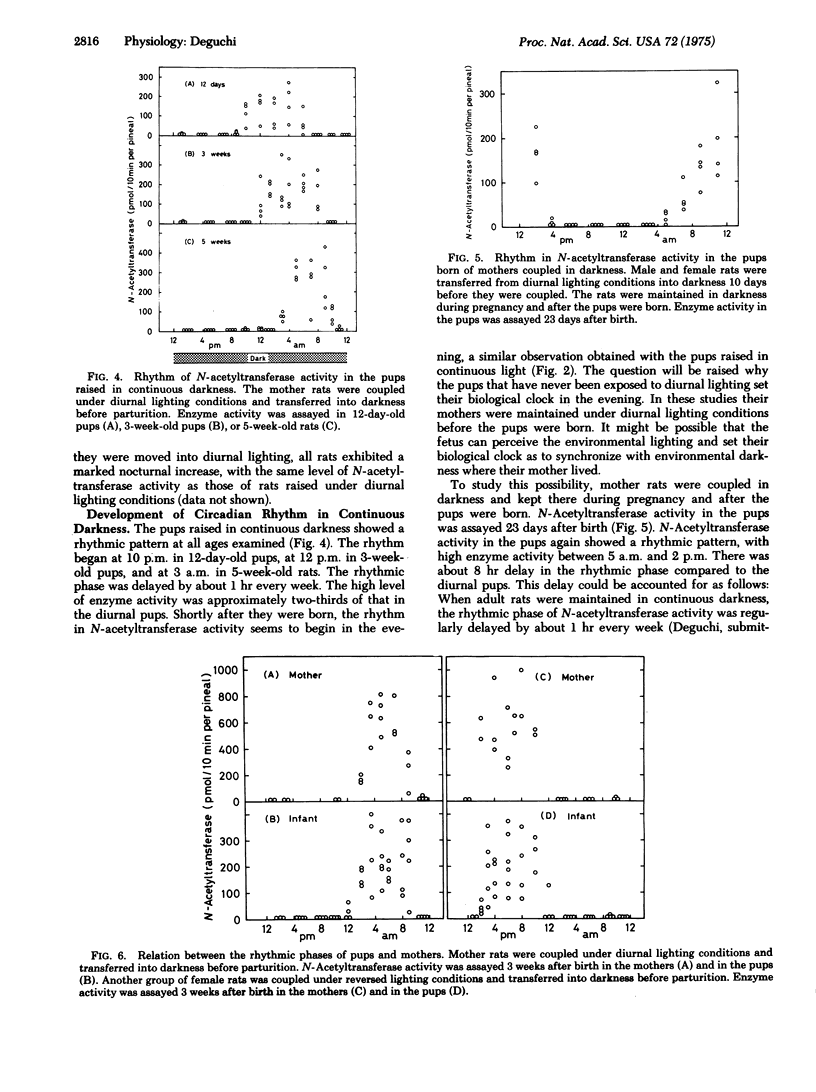
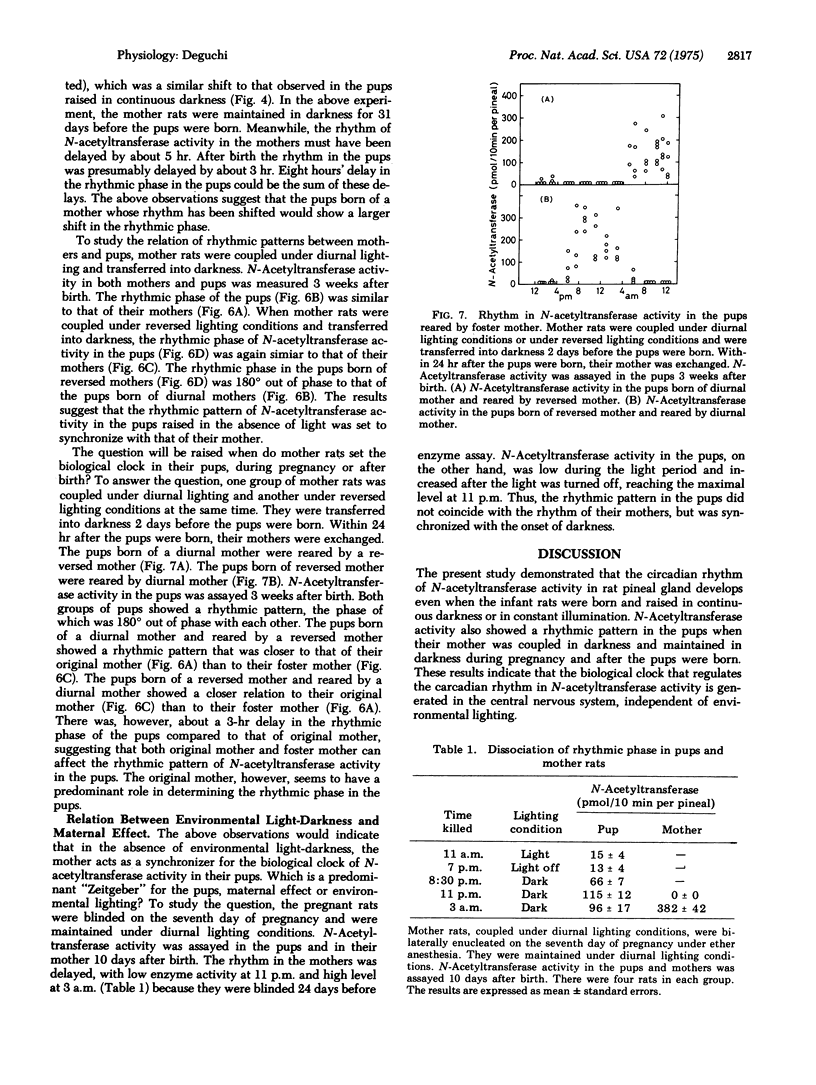
Serotonin:acetyl coenzyme A N-acetyltransferase (EC 2.3.1.5) Activity in pineal gland was assayed in rats which were born and raised under diurnal lighting conditions, in continuous darkness, or in constant light. N-Acetyltransferase activity in the pups raised under diurnal lighting showed a rhythmic pattern, with high enzyme activity during dark period. The pups raised in continuous darkness also showed a rhythmic pattern, the phase of which was delayed by 8 hr in 7-week-old pups; the rhythmic phase of N-acetyltransferase began in the evening in 12-day-old pups and was regularly delayed by 1 hr every week. The pups raised in constant illumination also showed a rhythmic pattern; the rhythmic phase was delayed by 3 hr every week. When the mother rats were coupled in darkness and maintained in darkness during pregnancy and after the pups were born, their pups again showed a rhythmic pattern. These observations indicate that the biological clock for N-acetyltransferase is generated independently of environmental lighting. When mothers were coupled under reversed lighting conditions and transferred into darkness or light, the rhythmic pattern in their pups was inverted 180 degrees from that of pups born of diurnal mothers. When the pups were raised in darkness, the rhythmic phase of N-acetyltransferase in the pups was similar to that of their mothers. It is suggested that in the absence of light-darkness cycle, the mother rat sets the rhythm of the pups to synchronize with her own rhythm. When pups were reared by a foster mother with a different rhythmic pattern from that of their original mother, the rhythmic phase in the pups was closer to that of the original mother, suggesting that the original mother plays the predominant role setting the rhythm of the pups.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod J. The pineal gland: a neurochemical transducer. Science. 1974 Jun 28;184(4144):1341–1348. doi: 10.1126/science.184.4144.1341. [DOI] [PubMed] [Google Scholar]
- Deguchi T., Axelrod J. Control of circadian change of serotonin N-acetyltransferase activity in the pineal organ by the beta--adrenergic receptor. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2547–2550. doi: 10.1073/pnas.69.9.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi T., Axelrod J. Sensitive assay for serotonin N-acetyltransferase activity in rat pineal. Anal Biochem. 1972 Nov;50(1):174–179. doi: 10.1016/0003-2697(72)90496-4. [DOI] [PubMed] [Google Scholar]
- Ellison N., Weller J. L., Klein D. C. Development of a circadian rhythm in the activity of pineal serotonin N-acetyltransferase. J Neurochem. 1972 May;19(5):1335–1341. doi: 10.1111/j.1471-4159.1972.tb01458.x. [DOI] [PubMed] [Google Scholar]
- Håkanson R., Lombard des Gouttes M. N., Owman C. Activities of tryptophan hydroxylase, dopa decarboxylase, and monoamine oxidase as correlated with the appearance of monoamines in developing rat pineal gland. Life Sci. 1967 Dec 15;6(24):2577–2585. doi: 10.1016/0024-3205(67)90107-5. [DOI] [PubMed] [Google Scholar]
- Klein D. C., Weller J. L. Indole metabolism in the pineal gland: a circadian rhythm in N-acetyltransferase. Science. 1970 Sep 11;169(3950):1093–1095. doi: 10.1126/science.169.3950.1093. [DOI] [PubMed] [Google Scholar]
- Klein D. C., Weller J. L., Moore R. Y. Melatonin metabolism: neural regulation of pineal serotonin: acetyl coenzyme A N-acetyltransferase activity. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3107–3110. doi: 10.1073/pnas.68.12.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R. Y., Klein D. C. Visual pathways and the central neural control of a circadian rhythm in pineal serotonin N-acetyltransferase activity. Brain Res. 1974 May 10;71(1):17–33. doi: 10.1016/0006-8993(74)90188-7. [DOI] [PubMed] [Google Scholar]
- QUAY W. B. CIRCADIAN RHYTHM IN RAT PINEAL SEROTONIN AND ITS MODIFICATIONS BY ESTROUS CYCLE AND PHOTOPERIOD. Gen Comp Endocrinol. 1963 Oct;3:473–479. doi: 10.1016/0016-6480(63)90079-0. [DOI] [PubMed] [Google Scholar]
- SNYDER S. H., ZWEIG M., AXELROD J., FISCHER J. E. CONTROL OF THE CIRCADIAN RHYTHM IN SEROTONIN CONTENT OF THE RAT PINEAL GLAND. Proc Natl Acad Sci U S A. 1965 Feb;53:301–305. doi: 10.1073/pnas.53.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISSBACH H., REDFIELD B. G., AXELROD J. Biosynthesis of melatonin: enzymic conversion of serotonin to N-acetylserotonin. Biochim Biophys Acta. 1960 Sep 23;43:352–353. doi: 10.1016/0006-3002(60)90453-4. [DOI] [PubMed] [Google Scholar]
- Zweig M., Snyder S. H., Axelrod J. Evidence for a nonretinal pathway of light to the pineal gland of newborn rats. Proc Natl Acad Sci U S A. 1966 Aug;56(2):515–520. doi: 10.1073/pnas.56.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]


