Abstract
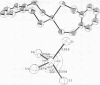
To complete the set of synthetic analogs of the three recognized types of active sites in iron-sulfur redox proteins, the compound (Et4N)[Fe((SCH2)2C6H4)2], derived from o-xylyl-alpha,alpha'-dithiol, has been prepared and its structure has been determined by x-ray diffraction. The bischelate anion contains a near-tetrahedral Fe(III)-S4 coordination unit with small rhombic distortions and all Fe-S bond distances in the range 2.252-2.279 A. Its electronic properties have been partially characterized by measurement of electronic absorption, paramagnetic resonance, Mössbauer spectra, and magnetic susceptibility. The analog, as the protein, exhibits the Fe(III)/Fe(II) redox couple. These results substantiate designation of [Fe((SCH2)2C6H4)2]- as a synthetic analog of the Fe(III)(S-Cys)4 center in oxidized rubredoxin proteins. Comparison of the analog structure with that of the Clostridium pasteurianum rubredoxin active site shows that the former is substantially less distorted from idealized tetrahedral symmetry, and is considered to represent an essentially unconstrained structural model of the latter. Provided the grossly distorted tetrahedral stereochemistry of the protein site persists through final structural refinement, the analog-protein structural comparison supports an entatic state description of oxidized rubredoxin.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adman E. T., Sieker L. C., Jensen L. H. Structure of a bacterial ferredoxin. J Biol Chem. 1973 Jun 10;248(11):3987–3996. [PubMed] [Google Scholar]
- Averill B. A., Herskovitz T., Holm R. H., Ibers J. A. Synthetic analogs of the active sites of iron-sulfur proteins. II. Synthesis and structure of the tetra(mercapto-m 3 -sulfido-iron) clusters, (Fe 4 S 4 (SR) 4 ) 2- . J Am Chem Soc. 1973 May 30;95(11):3523–3534. doi: 10.1021/ja00792a013. [DOI] [PubMed] [Google Scholar]
- Carter C. W., Jr, Kraut J., Freer S. T., Alden R. A. Comparison of oxidation-reduction site geometries in oxidized and reduced Chromatium high potential iron protein and oxidized Peptococcus aerogenes ferredoxin. J Biol Chem. 1974 Oct 10;249(19):6339–6346. [PubMed] [Google Scholar]
- Cerdonio M., Wang R. H., Rawlings J., Gray H. B. Letter: Spectroscopic and magnetic characterization of high potential iron-sulfur protein from Chromatium. J Am Chem Soc. 1974 Oct 2;96(20):6534–6535. doi: 10.1021/ja00827a057. [DOI] [PubMed] [Google Scholar]
- DePamphilis B. V., Averill B. A., Herskovitz T., Que L., Jr, Holm R. H. Synthetic analogs of the active sites of iron-sulfur proteins. VI. Spectral and redox characteristics of the tetranuclear clusters (Fe4S4(SR)4).2-. J Am Chem Soc. 1974 Jun 26;96(13):4159–4167. doi: 10.1021/ja00820a017. [DOI] [PubMed] [Google Scholar]
- Freer S. T., Alden R. A., Carter C. W., Jr, Kraut J. Crystallographic structure refinement of Chromatium high potential iron protein at two Angstroms resolution. J Biol Chem. 1975 Jan 10;250(1):46–54. [PubMed] [Google Scholar]
- Holm R. H. Iron-sulphur clusters in natural and synthetic systems. Endeavour. 1975 Jan;34(121):38–43. doi: 10.1016/0160-9327(75)90067-8. [DOI] [PubMed] [Google Scholar]
- Jacks C. A., Bennett L. E., Raymond W. N., Lovenberg W. Electron transport to clostridial rubredoxin: kinetics of the reduction by hexaammineruthenium(II), vanadous and chromous ions. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1118–1122. doi: 10.1073/pnas.71.4.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen L. H. X-ray structural studies of ferredoxin and related electron carriers. Annu Rev Biochem. 1974;43(0):461–507. doi: 10.1146/annurev.bi.43.070174.002333. [DOI] [PubMed] [Google Scholar]
- Koch S., Tang S. C., Holm R. H., Frankel R. H., Ibers J. A. Letter: Ferric porphyrin thiolates. Possible relationship to cytochrome P-450 enzymes and the structure of (p-nitrobenzenethiolato)iron(III) protoporphyrin IX dimethyl ester. J Am Chem Soc. 1975 Feb 19;97(4):916–918. doi: 10.1021/ja00837a052. [DOI] [PubMed] [Google Scholar]
- Lode E. T., Coon M. J. Enzymatic omega-oxidation. V. Forms of Pseudomonas oleovorans rubredoxin containing one or two iron atoms: structure and function in omega-hydroxylation. J Biol Chem. 1971 Feb 10;246(3):791–802. [PubMed] [Google Scholar]
- Lovenberg W., Sobel B. E. Rubredoxin: a new electron transfer protein from Clostridium pasteurianum. Proc Natl Acad Sci U S A. 1965 Jul;54(1):193–199. doi: 10.1073/pnas.54.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovenberg W., Williams W. M. Further observations on the chemical nature of rubredoxin from Clostridium pasteurianum. Biochemistry. 1969 Jan;8(1):141–148. doi: 10.1021/bi00829a020. [DOI] [PubMed] [Google Scholar]
- Mayerle J. J., Frankel R. B., Holm R. H., Ibers J. A., Phillips W. D., Weiher J. F. Synthetic analogs of the active sites of iron-sulfur proteins. Structure and properties of bis(o-xylyldithiolato-m2-sulfidoferrate (3)), an analog of the 2Fe-2S proteins. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2429–2433. doi: 10.1073/pnas.70.8.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme-Johnson W. H. Iron-sulfur proteins: structure and function. Annu Rev Biochem. 1973;42(0):159–204. doi: 10.1146/annurev.bi.42.070173.001111. [DOI] [PubMed] [Google Scholar]
- Peisach J., Blumberg W. E., Lode E. T., Coon M. J. An analysis of the electron paramagnetic resonance spectrum of pseudomonas oleovorans rubredoxin. A method for determination of the liganids of ferric iron in completely rhombic sites. J Biol Chem. 1971 Oct 10;246(19):5877–5881. [PubMed] [Google Scholar]
- Phillips W. D., Poe M., Weiher J. F., McDonald C. C., Lovenberg W. Proton magnetic resonance, magnetic susceptibility and Mössbauer studies of Clostridium pasteurianum rubredoxin. Nature. 1970 Aug 8;227(5258):574–577. doi: 10.1038/227574a0. [DOI] [PubMed] [Google Scholar]
- Que L., Jr, Anglin J. R., Bobrik M. A., Davison A., Holm R. H. Synthetic analogs of the active sites of iron-sulfur proteins. IX. Formation and some electronic and reactivity properties of Fe4S4 Glycyl-L-cysteinylglycyl oligopeptide complexes obtained by ligand substitution reactions. J Am Chem Soc. 1974 Sep 18;96(19):6042–6048. doi: 10.1021/ja00826a014. [DOI] [PubMed] [Google Scholar]
- Que L., Jr, Bobrik M. A., Ibers J. A., Holm R. H. Synthetic analogs of the active sites of iron-sulfur proteins. VII. Ligand substitution reactions of the tetranuclear clusters (Fe4S4(SR)4)2- and the structure of ((CH3)4N)2(Fe4S4(SC6H5)4). J Am Chem Soc. 1974 Jun 26;96(13):4168–4178. doi: 10.1021/ja00820a018. [DOI] [PubMed] [Google Scholar]
- Rao K. K., Evans M. C., Cammack R., Hall D. O., Thompson C. L., Jackson P. J., Johnson C. E. Mössbauer effect in rubredoxin. Determination of the hyperfine field of the iron in a simple iron-sulphur protein. Biochem J. 1972 Oct;129(5):1063–1070. doi: 10.1042/bj1291063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands R. H., Dunham W. R. Spectroscopic studies on two-iron ferredoxins. Q Rev Biophys. 1974 Nov;7(4):443–504. doi: 10.1017/s0033583500001517. [DOI] [PubMed] [Google Scholar]
- Tang S. P., Spiro T. G., Antanaitis C., Moss T. H., Holm R. H., Herskovitz T., Mortensen L. E. Resonance Raman spectroscopic evidence for structural variation among bacterial ferredoxin, HiPIP, and Fe4S4(SCH2Ph)42. Biochem Biophys Res Commun. 1975 Jan 6;62(1):1–6. doi: 10.1016/s0006-291x(75)80396-2. [DOI] [PubMed] [Google Scholar]
- Thompson C. L., Johnson C. E., Dickson D. P., Cammack R., Hall D. O., Weser U., Rao K. K. Mössbauer effect in the eight-iron ferredoxin from Clostridium pasterurianum. Evidence for the state of the iron atoms. Biochem J. 1974 Apr;139(1):97–103. doi: 10.1042/bj1390097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee B. L., Williams R. J. Metalloenzymes: the entatic nature of their active sites. Proc Natl Acad Sci U S A. 1968 Feb;59(2):498–505. doi: 10.1073/pnas.59.2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]