Abstract
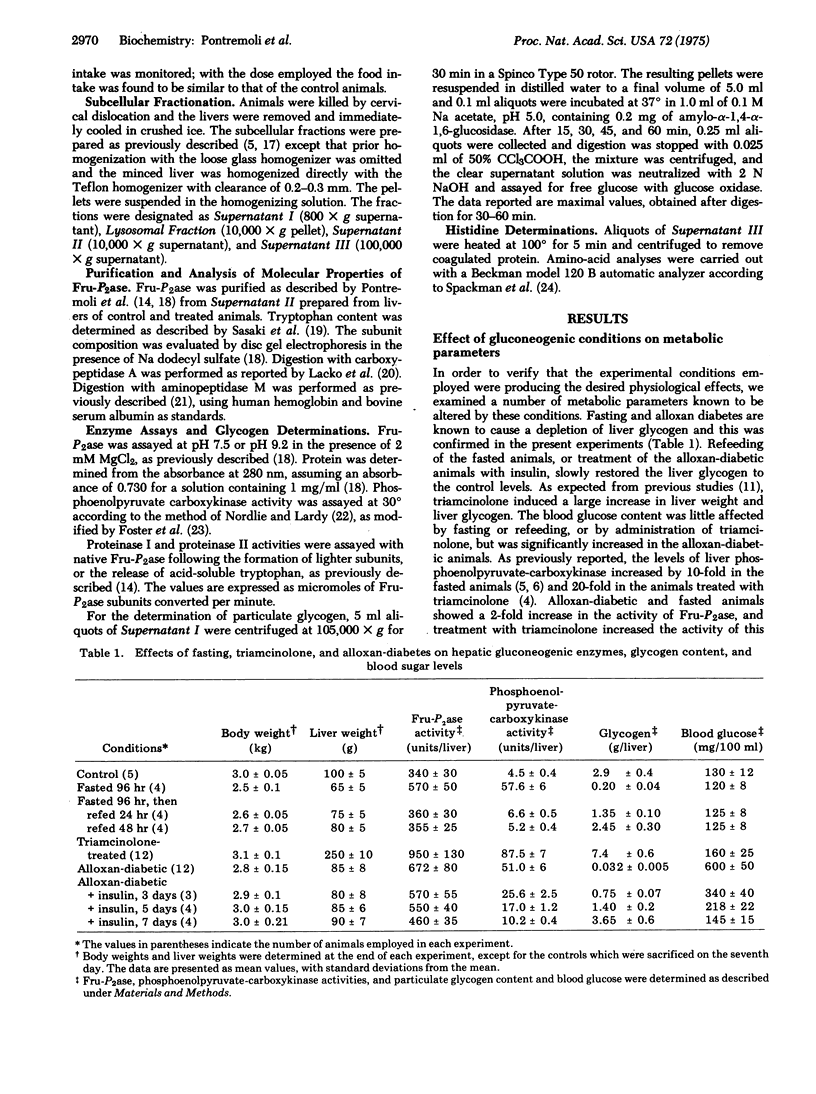
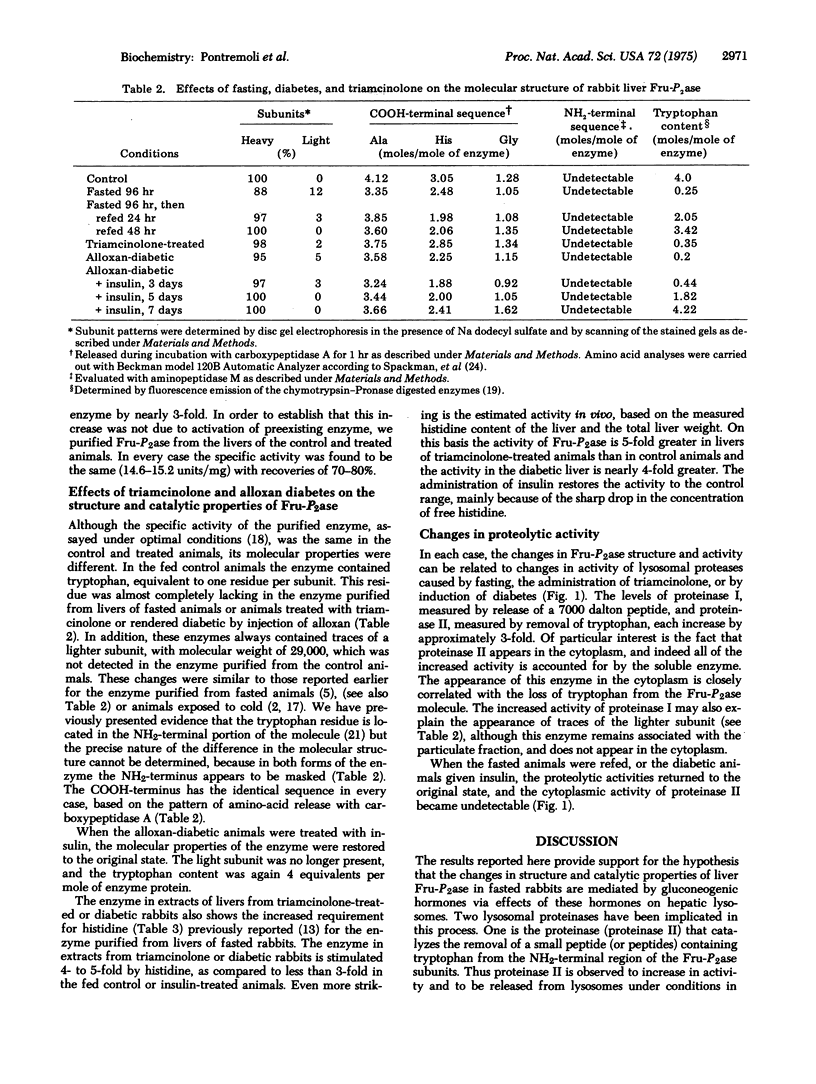
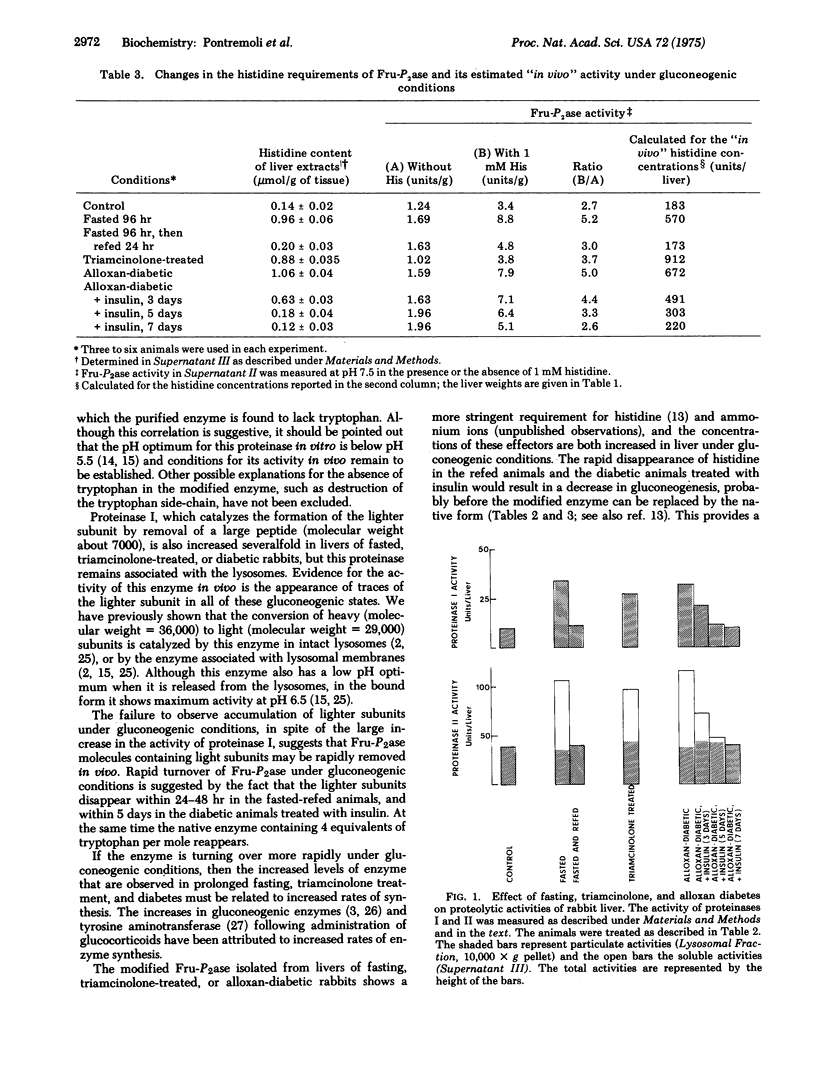
Gluconeogenic conditions, such as administration of triamcinolone or alloxan diabetes, cause the following changes in the molecular structure and properties of rabbit liver fructose 1,6-bisphosphatase (D-fructose-1,6-bisphosphate 1-phosphohydrolase, EC 3.1.3.11): (1) the appearance of traces (about 10%) of a lighter subunit; (2) loss of tryptophan from all of the subunits, including those that show no apparent change in molecular weight; (3) increase in requirement for the positive allosteric effector, histidine; (4) increase in amount of enzyme, but not its specific activity. These changes are identical to those induced by cold or fasting, and are related to increased activities of lysosomal proteases. The results suggest that lysosomes may act as mediators of gluconeogenic stimuli.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cahill G. F., Jr The Banting Memorial Lecture 1971. Physiology of insulin in man. Diabetes. 1971 Dec;20(12):785–799. doi: 10.2337/diab.20.12.785. [DOI] [PubMed] [Google Scholar]
- Deter R. L., De Duve C. Influence of glucagon, an inducer of cellular autophagy, on some physical properties of rat liver lysosomes. J Cell Biol. 1967 May;33(2):437–449. doi: 10.1083/jcb.33.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster D. O., Ray P. D., Lardy H. A. Studies on the mechanisms underlying adaptive changes in rat liver phosphoenolpyruvate carboxykinase. Biochemistry. 1966 Feb;5(2):555–562. doi: 10.1021/bi00866a022. [DOI] [PubMed] [Google Scholar]
- Howell S. L., Taylor K. W. The acute pancreatic effect of alloxan in the rabbit. J Endocrinol. 1967 Apr;37(4):421–427. doi: 10.1677/joe.0.0370421. [DOI] [PubMed] [Google Scholar]
- Johnson D. C., Brunsvold R. A., Ebert K. A., Ray P. D. Gluconeogenesis in rabbit liver. I. Pyruvate-derived dicarboxylic acids and phosphoenolpyruvate formation in rabbit liver. J Biol Chem. 1973 Feb 10;248(3):763–770. [PubMed] [Google Scholar]
- KAPLAN S. A., SHIMIZU C. S. Effects of cortisol on amino acids in skeletal muscle and plasma. Endocrinology. 1963 Feb;72:267–272. doi: 10.1210/endo-72-2-267. [DOI] [PubMed] [Google Scholar]
- Lacko A. G., Brox L. W., Gracy R. W., Horecker B. L. The carboxyl-terminal structure of rabbit liver aldolase (aldolase B). J Biol Chem. 1970 Apr 25;245(8):2140–2141. [PubMed] [Google Scholar]
- Lardy H. A., Foster D. O., Shrago E., Ray P. D. Metabolic and hormonal regulation of phosphopyruvate synthesis. Adv Enzyme Regul. 1964;2:39–47. doi: 10.1016/s0065-2571(64)80004-2. [DOI] [PubMed] [Google Scholar]
- NORDLIE R. C., LARDY H. A. Mammalian liver phosphoneolpyruvate carboxykinase activities. J Biol Chem. 1963 Jul;238:2259–2263. [PubMed] [Google Scholar]
- Pontremoli S., Accorsi A., Melloni E., Schiavo E., De Flora A., Horecker B. L. Transformation of neutral to alkaline fructose 1,6-bisphosphatase. Converting enzyme activity in the large-particle fraction from rabbit liver. Arch Biochem Biophys. 1974 Oct;164(2):716–721. doi: 10.1016/0003-9861(74)90084-8. [DOI] [PubMed] [Google Scholar]
- Pontremoli S., Melloni E., Accorsi A., De Flora A., Salamino F., Horecker B. L. Evidence for the modification of fructose 1,6-bisphosphatase by two distinct lysosomal proteases. Biochem Biophys Res Commun. 1974 Sep 9;60(1):474–481. doi: 10.1016/0006-291x(74)90228-9. [DOI] [PubMed] [Google Scholar]
- Pontremoli S., Melloni E., De Flora A., Horecker B. L. Conversion of neutral to alkaline liver fructose 1,6-bisphosphatase: changes in molecular properties of the enzyme. Proc Natl Acad Sci U S A. 1973 Mar;70(3):661–664. doi: 10.1073/pnas.70.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontremoli S., Melloni E., De Flora A., Horecker B. L. Regulation of fructose, 1,6-bisphosphatase by histidine under gluconeogenic conditions. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2166–2168. doi: 10.1073/pnas.71.6.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontremoli S., Melloni E., Salamino F., De Flora A., Horecker B. L. Changes in activity and molecular properties of fructose 1, 6-bisphosphatase during fasting and refeeding. Proc Natl Acad Sci U S A. 1974 May;71(5):1776–1779. doi: 10.1073/pnas.71.5.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontremoli S., Melloni E., Salamino F., Franzi A. T., De Flora A., Horecker B. L. Changes in rabbit-liver lysosomes and fructose 1,6-bisphosphatase induced by cold and fasting. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3674–3678. doi: 10.1073/pnas.70.12.3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T., Abrams B., Horecker B. L. A fluorometric method for the determination of the tryptophan content of proteins. Anal Biochem. 1975 May 12;65(1-2):396–404. doi: 10.1016/0003-2697(75)90524-2. [DOI] [PubMed] [Google Scholar]
- Smith O. K., Long C. N. Effect of cortisol on the plasma amino nitrogen of eviscerated adrenalectomized-diabetic rats. Endocrinology. 1967 Apr;80(4):561–566. doi: 10.1210/endo-80-4-561. [DOI] [PubMed] [Google Scholar]
- Sutherland E. W., 3rd, Haynes R. C., Jr Increased release of amino acids from rat thymus after cortisol administration. Endocrinology. 1967 Feb;80(2):288–296. doi: 10.1210/endo-80-2-288. [DOI] [PubMed] [Google Scholar]
- Traniello S., Melloni E., Pontremoli S., Sia C. L., Horecker R. L. Rabbit liver fructose 1,6-diphosphatase. Properties of the native enzyme and their modification by subtilisin. Arch Biochem Biophys. 1972 Mar;149(1):222–231. doi: 10.1016/0003-9861(72)90317-7. [DOI] [PubMed] [Google Scholar]
- Wagle S. R. Effects of insulin deficiency and cortisol administration on mobilization of protein to gluconeogenesis. Proc Soc Exp Biol Med. 1966 Apr;121(4):1297–1300. doi: 10.3181/00379727-121-31033. [DOI] [PubMed] [Google Scholar]
- Weber G., Singhal R. L., Stamm N. B., Lea M. A., Fisher E. A. Synchronous behavior pattern of key glycolytic enzymes: glucokinase, phosphofructokinase, and pyruvate kinase. Adv Enzyme Regul. 1966;4:59–81. doi: 10.1016/0065-2571(66)90007-0. [DOI] [PubMed] [Google Scholar]



