Abstract
Menopause is caused by changes in the function of the hypothalamic-pituitary-gonadal (HPG) axis that controls reproduction. Hypophysiotropic gonadotropin-releasing hormone (GnRH) neurons in the hypothalamus orchestrate the activity of this axis and are regulated by hormonal feedback loops. The mechanisms by which GnRH responses to the primary regulatory sex-steroid hormone, estradiol (E2) are still poorly understood in the context of menopause. Our goal was to determine whether the G protein-coupled estrogen receptor (GPER) is co-expressed in adult primate GnRH neurons, and whether this changes with aging and/or E2 treatment. We used immunofluorescence double labeling to characterize the co-expression of GPER in GnRH perikarya and terminals in the hypothalamus. Young and aged rhesus macaques were ovariectomized and given long-term (~2 year) hormone treatments (E2, E2 + progesterone, or vehicle) selected to mimic currently prescribed hormone replacement therapies used for the alleviation of menopausal symptoms in women. We found that about half of GnRH perikarya co-expressed GPER, while only about 12% of GnRH processes and terminals in the median eminence (ME) were double labeled. Additionally, many GPER labeled processes were in direct contact with GnRH neurons, often wrapped around the perikarya and processes and in close proximity in the ME. These results extend prior work by showing robust colocalization of GPER in GnRH in a clinically relevant model, and support the possibility that GPER-mediated E2 regulation of GnRH occurs both in the soma and terminals in nonhuman primates.
Keywords: GPER, GnRH, Aging, Monkey, Hypothalamus, Estrogen, Progesterone, Menopause, Median Eminence
INTRODUCTION
Menopause is often accompanied by one or more neurobiological symptoms such as hot flushes, depression, irritability, sleep disruptions, lowered energy levels, memory deficiency, sexual dysfunction, and cognitive decline [1–4]. The most common treatment for menopausal symptoms is hormone replacement therapy (HRT), used primarily to improve quality-of-life from vasomotor symptoms, vaginal dryness, and osteoporosis [5, 6]. However, the termination and subsequent revisitation of results of the Women’s Health Initiative (WHI) has resulted in considerable controversy about the relative benefits and risks of exogenous hormone therapies [7–11]. From the neurobiological perspective, further insights are needed into the complex mechanisms underlying reproductive aging and the effects of HRT on the brain, including the nature of cells that respond to hormones and whether/how this changes with aging.
In the mediobasal hypothalamus (MBH) of primates, gonadotropin-releasing hormone (GnRH) neurons synthesize a neuropeptide that is released from nerve terminals at capillaries within the median eminence (ME; [12–16]). GnRH secretion triggers the release of the gonadotropins from the anterior pituitary that in turn stimulates the production of sex steroid hormones, predominantly estradiol (E2) and progesterone (P4) in the ovaries. These hormones then feed back onto the hypothalamus and pituitary, ultimately modulating the production and release of GnRH. The anatomical site(s) of E2 feedback regulation onto GnRH neurons involves a combination of both direct and indirect inputs. Previously, E2 regulation of GnRH cells was thought to be entirely indirectly mediated by other neuronal cell types and/or glia, because GnRH cells do not co-express estrogen receptor (ER) α [17]. GnRH cells are now known to contain ERβ, STX-sensitive estrogen receptors, and G protein-coupled membrane estrogen receptor (GPER, sometimes called GPR30) [18–23]. Of these, membrane ERs enable GnRH cells to respond rapidly to E2 with increased action potential rate, intracellular Ca2+ oscillations, and GnRH peptide release [23–27].
This study focused on the co-expression of GPER in GnRH neurons, which has not been well characterized in primates, in the context of reproductive aging. To our knowledge, there has been only a single report showing co-localization of GPER on three GnRH neurons in the primate brain [23]; all other work in this arena was conducted ex vivo or electrophysiologically, and predominantly in the rodent model. Our laboratory recently reported age-related increases in the density of GPER-immunoreactive cells in the arcuate nucleus and periventricular nucleus of female rhesus monkeys and confirmed that GPER-positive cells are found in regions where GnRH neurons are expressed in the monkey [28]. Considering that GPER can mediate actions of E2 on GnRH cells, together with known aging-related changes in GnRH function in the macaque [29], we sought to address three questions: 1) Do GnRH neurons of adult monkeys co-express GPER? 2) In what cellular compartment (cell body, nerve terminal) is co-expression found? 3) How does this co-expression change with age or E2 treatment? Work was conducted in monkeys that were age-appropriate for the pre- and perimenopausal stages, and that received hormone treatments that more closely approximate current usage since the termination of the WHI.
METHODS
ANIMALS
A total of 39 rhesus monkeys (Macaca mulatta) of two age ranges were included in this study: young adult (n = 17; aged 9.9 yr ± 2.3 yr) and aged (n = 22; aged 22.6 yr ± 1.6 yr). Young animals were all premenopausal and aged animals were all perimenopausal, as determined by normal cycling lengths of 24-34 days, or irregular cycles longer than 35 days, respectively [30]. Cycle length was established by daily visual inspection of vaginal bleeding for 2 years before initiation of the study. Additionally, all of the animals in the aged group were over 19 years because prominent endocrine and neurobiological signs of aging do not occur in monkeys before reaching that age [31]. The animals were housed and experiments carried out at the California National Primate Research Center, as described previously [32–37]. Procedures were approved by the Institutional Animal Care and Use Committee at the University of California and in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All of the monkeys were selected based on the following criteria: they had no long-term or invasive dietary or pharmacological interventions, and they were all in good physical health with no physical abnormalities or severe arthritis.
OVARIECTOMY & HORMONE TREATMENT
All animals were ovariectomized (OVX) as previously described [32–37]. Briefly, they were sedated with ketamine (10 mg/kg) and atropine (0.4 mg/kg), anesthetized with isoflurane, and their ovaries and Fallopian tubes removed. They were given a 2-3 month recovery period before assignment to one of the three treatment groups, designed to mimic current hormone regimens commonly prescribed to women. These consisted of the following conditions: vehicle (young n = 7 [YV], aged n = 8 [AV]), continuous estradiol (young n = 5 [YE], aged n = 6 [AE]) and continuous estradiol plus cyclic progesterone (young n = 5 [YEP], aged n = 8 [AEP]). The duration of all hormone or vehicle treatments were 2 years. Some animals were excluded from the study due to illness or incomplete OVX as determined by hormone analysis; the n's reported here are the final numbers used in this study. For the hormone groups, two Silastic capsules (3 cm long and 0.46 inner diameter), containing crystalline estradiol (E2 Sigma) were implanted subcutaneously between the shoulder blades, achieving a mean serum level of 150 pg/ml, and were replaced every three months. The vehicle group received empty capsules on the same schedule, plus an intramuscular injection of 1 ml peanut oil to mimic a fourth treatment group (cyclic estradiol) that we were unable to include in this study due to the lack of aged-matched animals. The E2 + cyclic P4 group received 100 mg of micronized progesterone (Catalent Pharm Solutions), mimicking luteal phase P4 levels, in their daily fruit treat, for 10 out of every 28 days, while the other groups received untreated fruit.
EUTHANASIA AND TISSUE PROCESSING
The monkeys were perfused 8-10 days after the last P4 treatment and on a comparable day for the other (non-P4) groups as previously described [38, 39]. Briefly, they were deeply anesthetized with ketamine (25 mg/kg) and pentobarbital (20–35 mg/kg) and were perfused transcardially with ice-cold 1% paraformaldehyde, followed b y 4% paraformaldehyde in phosphate buffer (PB), for 1 minute and 12 minutes (at 185 ml/min), respectively. The brain was removed and the hypothalamus was dissected then postfixed for 6 hours in 4% paraformaldehyde and 0.125% glutaraldehyde in PB and shipped to the University of Texas at Austin in PB for further processing. The mediobasal hypothalamus (MBH) was sectioned at 100 µm using a vibrating microtome (Leica VT1000 S; Leica, Bannockburn, IL). Half of the median eminence (ME), when present, was dissected for use in companion studies and the remaining sections were cryoprotected in a sucrose series (12%, 18%, 30% for 48 hrs each) and stored at -20 °C in 30% glycerol for later use.
IMMUNOHISTOCHEMISTRY
We used dual-label fluorescence immunohistochemistry (IHC) to visualize GnRH and GPER distribution in the MBH. Four to five sections containing the MBH were collected from each animal and allocated into rostral, medial and caudal groups for IHC. There were an uneven number of sections available from each region due to tissue damage and loss from poor perfusions and processing errors. A second IHC run was performed to fill in gaps from animals in the first run in which fewer than 5 GnRH neurons were found. Between each step of the IHC, sections were washed in phosphate buffered saline (PBS; pH 7.4) 3×10 minutes. Due to the thickness of the sections, we used a citrate buffer (pH 8.5; preheated to 70 °C; 30 min) for antigen retrieval. Nonspecific binding sites were blocked with a solution of 10% normal goat serum (NGS; S-1000, Vector Laboratories, Burlingame, CA, USA) and 2% bovine serum albumin (BSA; Sigma-Aldrich, USA) for 90 minutes. We incubated sections for 48 hours (at 4°C with constant agitation) in mouse anti-GnRH (HU11b, diluted to 1:1,000; a gift from Dr Henryk Urbanski) and rabbit anti-GPER primary antibody (diluted 1:1,000; a gift from Dr. Edward Filardo), in 2% NGS in PBS. We then incubated the sections simultaneously in the following secondary antibodies for 2 hours: Alexa Fluor 594 conjugated goat anti-mouse IgG and Alexa 488 anti-rabbit IgG (both diluted 1:400; Life Technologies, Eugene, OR, USA), in a solution of 5% NGS in PBS. The primary antibodies have been thoroughly validated in rodent and primate tissue [22, 23, 40–45]. Here, the primary antibodies were omitted in experimental controls and no specific labeling for either fluorophore was found. To reduce lipofuscin autofluorescence, we treated the tissue with 10 mM CuSO4 in 50 mM ammonium acetate for 20 minutes. The sections were then mounted on glass slides, allowed to dry for 10 minutes and coverslipped using Vectashield mounting medium (Vector Laboratories, Burlingame, CA). The edges of the coverslip were sealed with clear nail polish. The slides were stored light-protected at 4°C.
CONFOCAL MICROSCOPY
We used a Zeiss LSM 710 microscope running Zen Black software (Carl Zeiss International, version date: 2012) with a Plan-Apochromat 40x/1.4 Oil DIC M27 objective for imaging the MBH of each animal. At the beginning of each imaging session, the laser power, gain and offset were adjusted using the negative control as a reference. For imaging the perikarya and ME, the two fluorophores were scanned sequentially to prevent signal bleed-through and the average of two scans for each line was recorded to decrease background. The GnRH scan was done with an excitation wavelength 561 nm (detection range 566 nm – 689 nm) followed by GPER with an excitation wavelength 458 nm (detection range 492 nm – 598 nm). To map the distribution of GnRH immunoreactive (IR) cell bodies, we imaged the entire MBH plus some surrounding regions where hypophysiotropic GnRH perikarya are distributed [46, 47]. GnRH neurons are 5-10 µm in thickness and to ensure that no GnRH perikarya would be missed, we took a z-stack with a voxel depth of 5 µm and a step size of 9 µm through the whole section. GnRH neurons were detected through the entire thickness of the sections, indicating that there was complete penetration of the antibodies. The location of each GnRH-IR neuron was marked as single (GnRH only) or double (GnRH+, GPER+) on a diagram of the MBH representing the rostral, medial and caudal levels based on four to six overlapping slices (depending on neuron orientation; with voxel depth of 2 µm) through each neuron for GnRH-IR followed by GPER-IR. To determine the relationship between GPER-IR and GnRH neuroterminals in the ME, we took a high-resolution image stack of the internal and external zones of 1-2 ME sections (depending on tissue availability) for each animal.
ANALYSIS & STATISTICS
All perikarya were analyzed with Fiji software [48], and MatLab software (version R2013a; MathWorks, Natick, MA) was additionally used for calculating the percent colocalized puncta in the ME. We used 2 methods in concert to categorize the immunoreactivity of the perikarya, both the traditional pseudocolored merge of the 2 channels and a grey-scale analysis to control for any optical illusions due to color perception bias. This was done with an image calculator plug-in in Fiji that created a single gray- scale image that shows the minimum intensity for each pixel of the 2 channels (i.e. if there is no colocalization then the minimum intensity is black). We rated the amount of GPER-IR, from 1-6, in each GnRH-IR perikarya with both methods, and re-scored a random subset of the images to ensure consistency. The scores from the two categorization methods were compared, resulting in three categories: no GPER-IR, moderate/punctate colocalization, and heavy colocalization.
We used R statistical packages (R Development Core Team, 2012) for all statistical analyses. Kruskal-Wallis (p) nonparametric test were used because our dataset was not normally distributed as was determined by the Shapiro-Wilks normality and Levene's equality of variance tests. Our alpha level was set to determine significant main effects (p ≤ 0.05) or trends (0.05 < p < 0.10) followed by a pairwise Wilcoxon post-hoc test to identify specific interactions [49, 50].
RESULTS
GnRH perikarya in the MBH co-express GPER
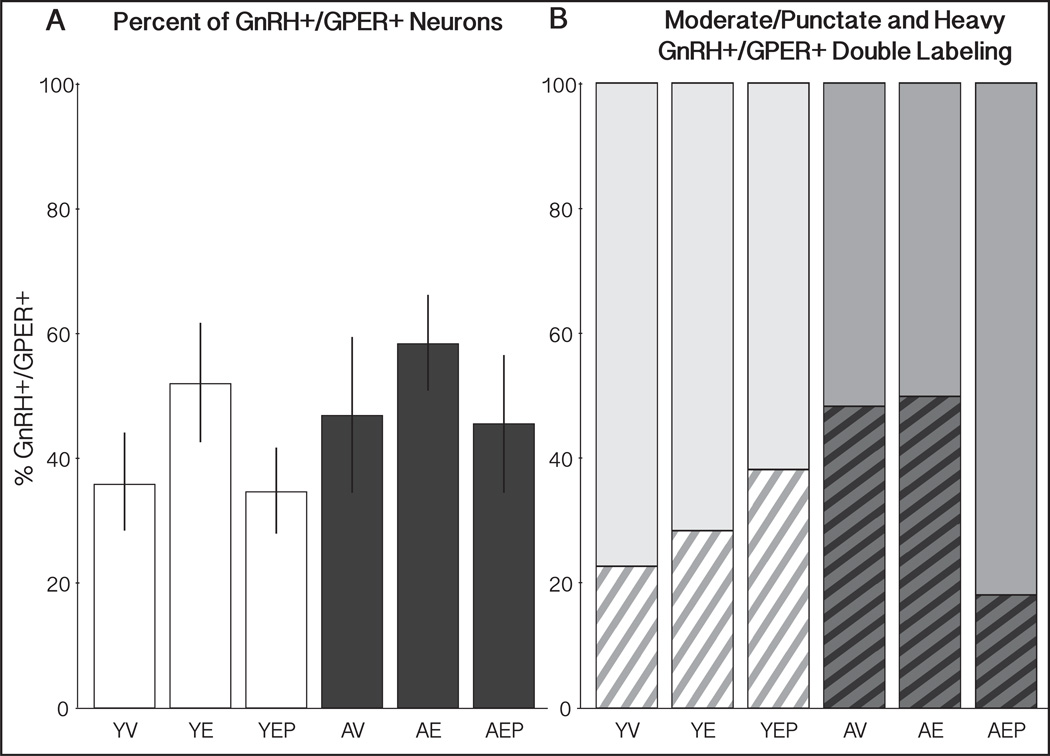
Examples of GnRH perikarya in the MBH that did or did not co-express GPER are shown in Figure 1. GnRH neurons in the 6 groups of monkeys (YV, YE, YEP, AV, AE, AEP) had the characteristic morphology of bipolar or unipolar perikarya with large nuclei, and were scattered through the basal hypothalamus. No significant main effects of age or hormone treatment were found for the morphology or average number of GnRH neurons per section (data not shown). For double labeling, about half of GnRH-IR neurons expressed GPER, regardless of age or hormone treatment (Figure 2A). Labeling of GPER in the GnRH neurons was variable, with some GnRH cells showing punctate or moderately intense labeling and others with very heavy labeling throughout the cytoplasm and into the varicosities (Figures 1 & 3). Additionally, we observed many GPER-IR processes in close contact with GnRH perikarya and/or proximal processes (Figure 3). During analysis we noticed great variability in the intensity of GPER immunolabeling in GnRH neurons. Therefore, we further subdivided double-labeled GnRH cells qualitatively into moderate/punctate or heavy GPER co-localization. There were no significant effects of age or treatment on the percent or type of double-labeled GnRH+/GPER+ neurons (Figure 2B).
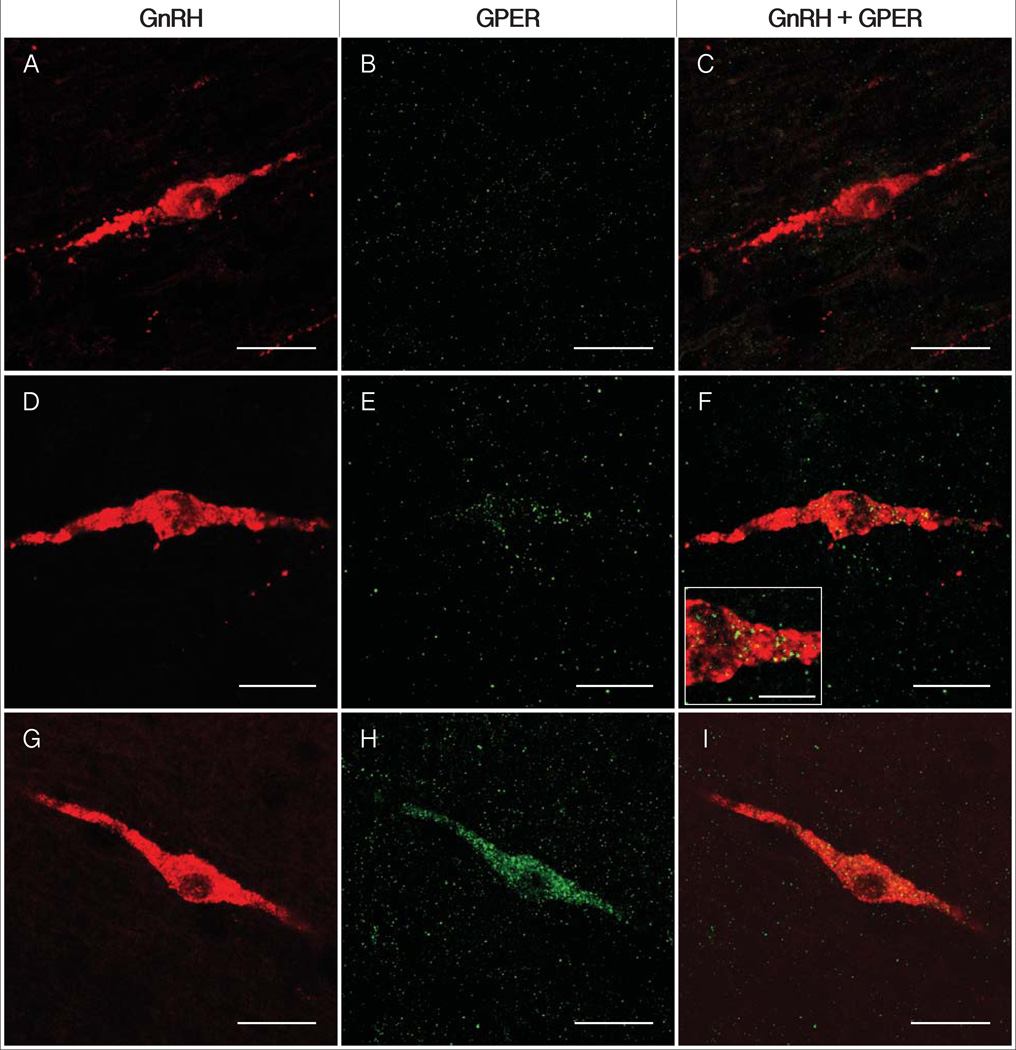
Figure 1.
Photomicrographs of GnRH-IR (left column), GPER-IR (center column) and double labeling (right column). Representative micrographs of the categories of GPER (green) expression within GnRH neurons (red) are shown for unlabeled (A-C), moderate / punctate GPER labeling within GnRH perikarya (D-F), and heavy cytoplasmic double labeling (G-I). The high magnification inset (F) shows punctuate GPER-IR. Scale bars = 25 µm (A-I), 10 µm (F inset).
Figure 2.
Percent of GnRH perikarya that express GPER (GnRH+/GPER+) and the subset of those double-labeled cells that have heavy cytoplasmic label. (A) The average percent (± SEM) of GnRH+/GPER+ perikarya throughout the entire MBH for young (white bars) and aged (black bars) monkeys is shown. (B) The percent of GnRH+/GPER+ cells with either moderate/punctate (top, solid) or heavy cytoplasmic (bottom, striped) GPER-IR, for each group. There were no significant effects of age or treatment in the total percent or type of double-label.
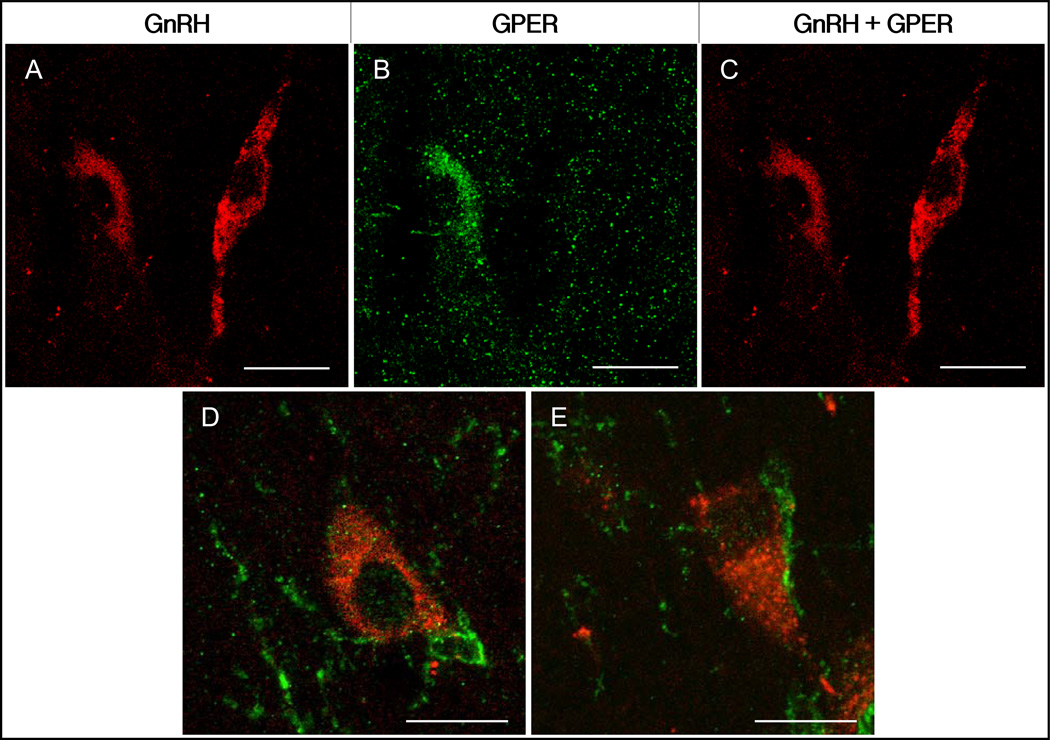
Figure 3.
Photomicrographs of double labeling of GnRH perikarya, and the proximity between GPER processes and GnRH neurons. Panels A-C show two GnRH neurons, one heavily colocalized with GPER (left) next to one that is not labeled with GPER (right). Examples of GnRH perikarya (D & E, red) that are in direct contact with GPER-IR processes (green). Scale bars = 35 µm (A-C), 15 µm (D & E).
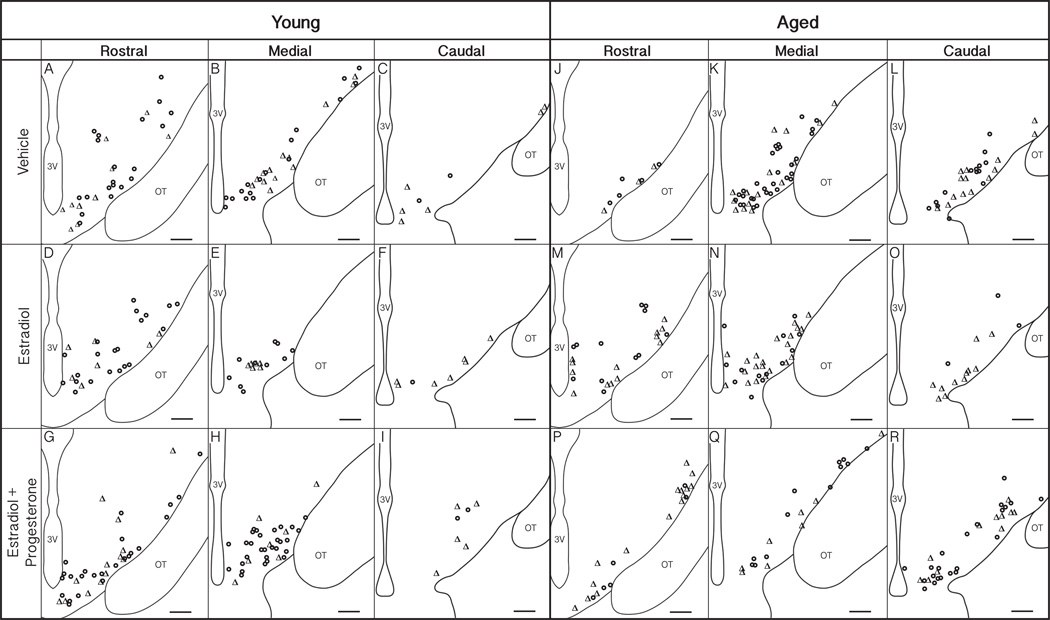
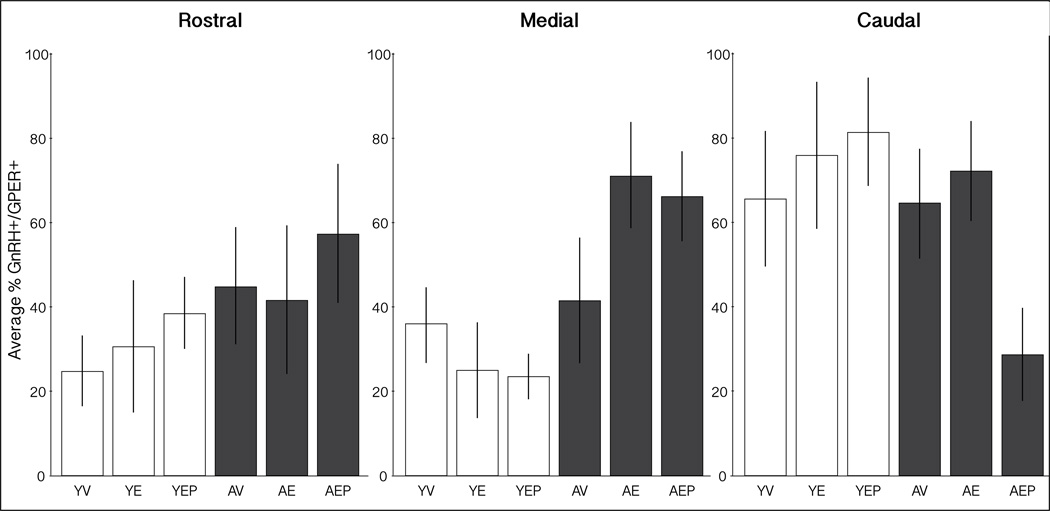
The distribution of GnRH neurons that were single (GnRH+/GPER−) or double (GnRH+/GPER+) labeled was mapped onto representations of the MBH from rostral to caudal, separately for the 6 groups (Figure 4). The mean percentage of colocalized neurons was also calculated across the three regions (Figure 5). In the medial MBH, there was a trend for an effect of age (p = 0.08), with aged animals tending to have a higher percentage of GnRH+/GPER+ than young groups. In the caudal MBH there were trends for effects of both age and treatment (p = 0.06, p = 0.09, respectively).
Figure 4.
Distribution of GnRH neurons in the medial basal hypothalamus (MBH). GnRH neurons were mapped onto a representation of coronal sections of the MBH from rostral (A, D, G – young; J, M, P –aged), medial (B, E, H – young; K, N, Q – aged) and caudal (C, F, I – young; L, O, R – aged) in young (left) and aged (right) adult rhesus monkeys of the three hormone treatment groups. GnRH+/GPER+ double-labeled perikarya are labeled with Δ and GnRH+/GPER- with o. The third ventricle (3V) and optic tract (OT) are labeled for orientation. Scale bars = 350 µm.
Figure 5.
Distribution of GnRH+/GPER+ cells in the MBH. These graphs show the average percent (±SEM) of GnRH+/GPER+ perikarya in the rostral (left), medial (middle) and caudal (right) MBH. There were trends of age effects in the medial and caudal regions (p=0.08 and p=0.06, respectively), as well as a treatment effect in the caudal MBH (p=0.09).
GnRH fibers have low co-expression of GPER in the median eminence
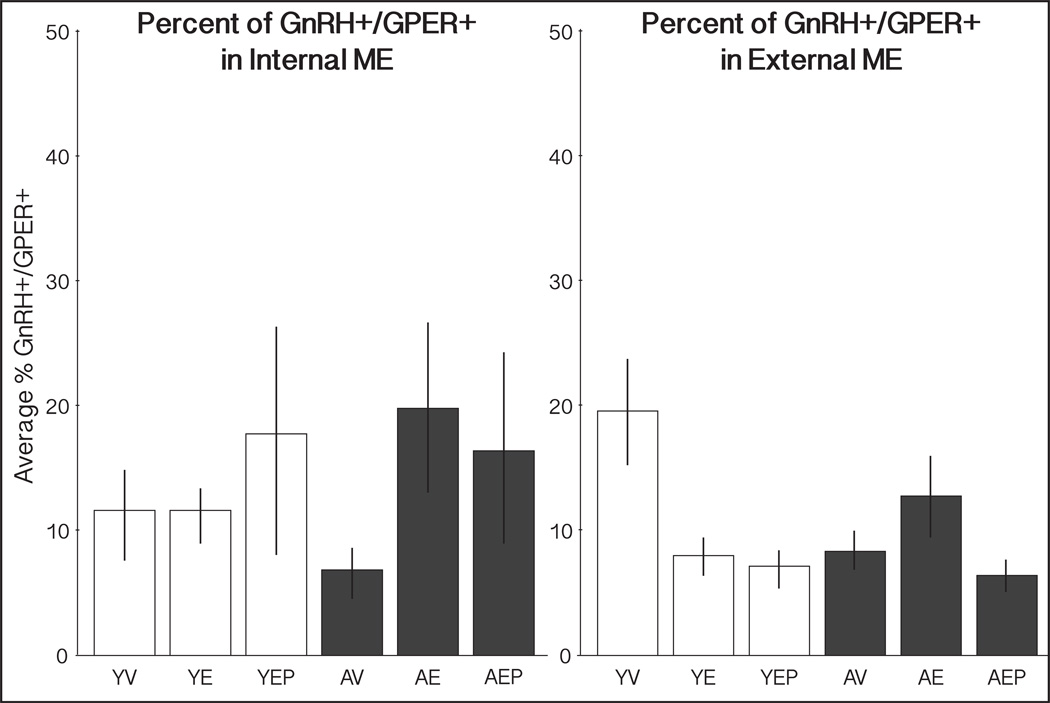
Both GnRH and GPER were detectable as punctate labeling in the ME (Figure 6). Co-localization of GPER in GnRH+ neural puncta was quantified separately in the internal and external zones of the ME. In both regions there was an average of 12% colocalization of GnRH-IR and GPER-IR puncta. There were no significant effects of age or treatment (Figure 7).
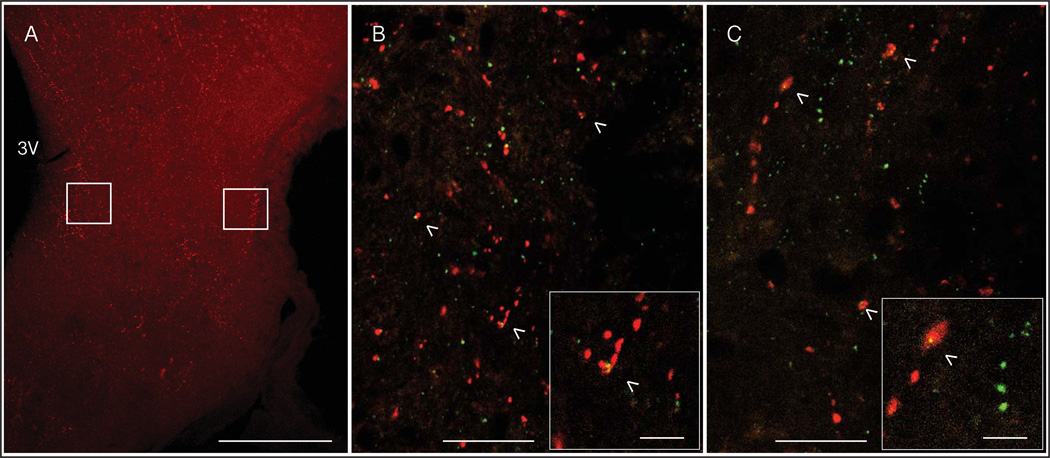
Figure 6.
Representative micrographs of GnRH and GPER immunolabeling in the ME. A low magnification image of GnRH-IR in a coronal section through the ME is shown (A) with boxes around the regions of the internal (left) and external (right) zones that were analyzed. The third ventricle (3V) is labeled for orientation. High magnification micrographs of punctate GnRH-IR (red) and GPER-IR (green) are shown in the internal (B) and external (C) zones of the ME. Colocalized puncta (yellow) are indicated with arrowheads. The insets show a high magnification example of colocalization in each region. Scale bars = 350 µm (A), 20 µm (B & C), 5 µm (insets).
Figure 7.
Percent colocalization of GnRH+/GPER+ puncta in the ME. The mean percent (±SEM) of GnRH puncta that were colocalized with GPER-IR in the internal and external ME are shown for each group. There were no significant effects of age or treatment in either region.
DISCUSSION
The goal of current work was to add to limited knowledge about expression of GPER, a membrane estrogen receptor, on hypophysiotropic GnRH neurons. We sought to gain novel insights into whether estrogenic regulation may occur directly on GnRH neurons through co-expression, or indirectly via appositions, in a non-human primate model. Prior work, mainly conducted ex vivo, has largely supported the possibility of coexpression, but few studies have investigated this relationship in a physiological system. Our current data adds three novel pieces of information. First, we ascertained that GnRH cells co-express GPER in adult rhesus monkeys; second, we determined the localization of this co-expression on cell bodies and processes/terminals in the ME; and third, we investigated whether aging and long-term hormone treatments affected co-expression in a clinically-relevant rhesus macaque model of menopause.
GnRH perikarya co-express GPER
The expression of GPER in the brain, including the hypothalamus, is quite abundant in primates and rodents, though there have been few studies that have examined GPER in GnRH neurons [28, 51–54]. Here, using hypothalami from OVX monkeys of two ages and three hormone treatments, we found that approximately half of the GnRH perikarya in the MBH were GPER-immunoreactive. Although there were some trends for effects of age and hormone treatment, none were robust, suggesting that co-localization of GPER in GnRH neurons of adult female monkeys is a stable part of the GnRH phenotype. All of the studies that have examined GPER in the context of primate GnRH neurons support co-localization but results in rodents are inconsistent. More specifically, cultured embryonic monkey GnRH neurons and GnRH GT1-7 cell lines co-express GPER [21, 23, 27]. In mice, Sun et al. reported that about one-third of GnRH neurons were electrophysiologically activated by the GPER agonist, G1 [19]. However, conflicting results were found by Romano et al., who did not detect rapid responses to G1 in any of the seven mouse GnRH neurons examined [55]. There may be species differences in the estrogenic regulation of GnRH via GPER. Additionally, alternate pathways, involving the classical receptors ERα and ERβ, that mediate rapid E2 activation of GnRH have been found in mice that are not present in primates [19, 21, 23, 25, 55–57]. For example, Abraham et al. discovered that ERβ induced CREB phosphorylation in the GnRH neurons, and Romano et al. found an ERα-dependent pathway involving GABAergic modulation of GnRH Ca2+ dynamics [25, 55]. While rapid E2 activation of primate GnRH neurons can occur independently of either of these classical ERs [20, 21]. Thus, the role that these ERs play in the regulation of GnRH in primates likely differs from rodents and further research is merited to resolve these differences.
The functional role of GPER in GnRH neurons is not known, although it has been suggested that it may mediate some aspects of hormone feedback and/or synchronization required for pulsatile release [58]. The lack of an obvious reproductive phenotype of the GPER-knockout mice may have hindered research in this area, as the global knockout may have developmental compensation for physiological roles normally mediated by GPER and, as discussed above, the role of GPER may be different in primates [18, 22, 59, 60]. There are still substantial gaps in knowledge about the receptors that mediate positive and negative effects of estrogens on GnRH neurons, making the GPER of potential interest beyond ERα, ERβ and STX-sensitive receptors [22, 57, 61]. Although at the time experiments were conducted it was not possible to perform pharmacological work on our monkeys, future work should investigate effects of E2 action on GnRH neurons in the presence of agonists or antagonists to GPER.
The relationship between GnRH perikarya and GPER is highly heterogeneous
Our analyses revealed that the intensity and pattern of GPER-IR varied between individual GnRH neurons: some cells had exclusively punctate and sparse labeling, while others had GPER-IR distributed densely and evenly throughout the cell. Many of the labeled GnRH perikarya in these monkeys showed GPER labeling in proximal processes and, in a few cases, more distal from the GnRH soma. Consistent with our results, GPER has been found in multiple intracellular compartments, including mitochondrial membranes [62], the endoplasmic reticulum and Golgi apparatus [63, 64], near the nucleus [64, 65], throughout the cytoplasm [51, 53, 62], as well as on the cell membrane [23, 28, 51, 62, 65], in a tissue and species-specific manner. The physiological roles that these compartmental differences may play are currently unknown.
Some GnRH neurons, while not double-labeled, were found in very close association with GPER-IR varicosities. In many cases, the GPER-IR processes appeared to wrap around GnRH soma, proximal dendrites or axons. Electron microscopy would be needed to verify whether or not these closely apposed cells have synapses or gap junctions, but if that were the case, then GPER+ cells may also mediate effects of E2 indirectly onto the GnRH neurons they contact. These GPER-IR processes could arise from cells that are either neuronal or glial, as GPER has been found in both types of cells in the regions near GnRH perikarya [62]. Together, these results of co-expression and close proximity to GnRH suggest that GPER could potentially mediate both direct and indirect E2 regulation of GnRH neural activity at or near the soma.
We found some regional specificity in the rostral to caudal distribution of GnRH+/GPER+ neurons. In young monkeys, more GnRH neurons were detected in the rostral and medial MBH, while in aged animals they were located more mediocaudally. Due to limitations in the numbers of animals and tissues we do not want to overinterpret these data. Nevertheless, consistent with our results, age-related changes in the distribution of GnRH cells in rodents have been reported [44, 66]. Rubin et al. found that there was an increase GnRH neurons in the dorsomedial portion of the preoptic area of young rats preceding the LH surge and this was not observed in middle-aged animals [66]. Additionally, Miller et al. found that there were fewer GnRH neurons in the medial septum of middle-aged irregular and acyclic rats in comparison to young or regularly cycling rats [44]. Together these studies suggest that the distribution of GnRH-IR perikarya may be responsive to the hormonal milieu, and age-related alterations in the dynamics of this system may be linked to reproductive senescence in rodents. While the differences in distribution that we measured did not reach significance, the results found here are suggestive of the possibility that monkeys also undergo age-related reorganization of GnRH perikarya.
We did not find any obvious change in GnRH cell number, consistent with observations in monkeys, mice, and rats [44, 46, 67, 68]. Although a few others have found some age-related changes in number of GnRH neurons in aged rats and mice, these were relatively small effects [66, 69]. Thus, the literature as a whole suggests small to no changes in GnRH perikarya numbers with aging, but possible distributional changes.
GnRH terminals in the ME have low co-expression of GPER
The median eminence is an important site of GnRH regulation [70–72]. The external zone of the ME contains the portal capillary bed and has a high density of secretory neuroterminals and tanycytic endfeet and is the location of GnRH release. The internal zone of the ME contains GnRH processes and the cell bodies of tanycytes, specialized glial cells that form a selective barrier to the portal capillaries [70, 73–74]. Age-related changes to GnRH release measured in the ME have been reported in rodents, in which release was decreased, and in monkeys, in which GnRH pulses (especially amplitude) were increased [29, 75]. The ME region undergoes dramatic cytoarchitectural and phenotypic changes with reproductive aging and hormone treatment in rats, especially around the terminals and capillaries, which directly affect the release of GnRH [70–74, 76–78]. Species differences in GnRH release are, in part, attributable to feedback regulatory differences with aging, underscoring the importance of conducting this work in a non-human primate model.
In the current study, we found similar amounts of colocalization of GPER and GnRH in the internal and external zones of the ME, with no age or treatment effects. There was a much lower percent of colocalization in the ME (~12%), in comparison to the perikarya of GnRH neurons (~50%). There are several interpretations for these cell compartment differences that are not mutually exclusive. GnRH perikarya may transport only a small percentage of synthesized GPER along the axon to the nerve terminals and processes in the ME. Alternatively or in addition, the detectability of GPER in the ME may be below the threshold of sensitivity of confocal microscopy, something that would require electron microscopy to resolve. Confirming our finding of GPER in the monkey ME, a recent study conducted by our laboratory in a model of natural reproductive aging in the rat also showed that GPER mRNA is detectable in the microdissected ME (inclusive of both internal and external zones). Furthermore, in that rat study, GPER mRNA expression was positively correlated with the genes for ERα, TGFα, prodynorphin, the vitamin D receptor, and the progesterone receptor, and was negatively correlated with the GABA-B2 receptor [79]. While follow up work is needed to further study any functional relationships, these data suggest some interesting molecular candidates for a regulatory network of GnRH in the ME, among which is GPER.
Summary and limitations
In this study conducted in young and aged OVX rhesus macaques, about half of GnRH perikarya express GPER, whereas a smaller percentage of processes and terminals (12%) had co-expression in the ME. GnRH cell bodies and processes were often in close apposition or proximity to GPER-immunoreactive elements. These data add new information about the robust presence of GPER in and around GnRH cells and suggest a viable site of direct action of estrogens on the GnRH system. Additionally, aging and hormone treatments had limited effects on the co-expression of GnRH and GPER in the rhesus monkey model of menopause. This latter result underscores the stability of GnRH neurons across adult development and into aging.
As is often the case for studies of non-human primates, we had a number of experimental constraints. The number of animals that were available for this experiment, and the sometimes-poor quality of tissues, limited the statistical power. In addition, there are both strengths and weaknesses to the OVX +/− hormone treatment model. While substantial numbers of women undergo surgical menopause, by far the majority of women undergo a natural menopause, involving a more gradual loss of ovarian hormones. It was not possible to utilize ovary-intact monkeys for the current study, as rhesus macaques undergo reproductive senescence very late in life [30] and such elderly monkeys were not attainable. However, we would also like to underscore some advantages of our model. Our young and aged monkeys corresponded roughly to young adult (~30 years) and middle-aged adult (~50 years) women. Thus, work was conducted in age-appropriate animals in terms of chronological years. In addition, as a primary goal was to gain insights into how hormone treatments affect neurobiological responses, the hormone deprivation or replacement models enabled us to directly address this point not only in our study but in our collaborators’ laboratories, who also utilized these valuable brain tissues [32–39]. In ongoing work we are further delving into additional biomedically-relevant models of the timing and duration of hormone replacement relative to deprivation in a test of the “critical window” hypothesis of menopause [6].
Table 1.
Group Numbers of GnRH Perikarya, GnRH+/GPER+ Perikarya and Sections for the Three Regions
| Total # GnRH Perikarya | Total # GnRH+/GPER+ Perikarya | Total # Sections | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Rostral | Medial | Caudal | Rostral | Medial | Caudal | Rostral | Medial | Caudal | |
| YV | 33 | 33 | 11 | 7 | 12 | 8 | 16 | 10 | 11 |
| YE | 37 | 22 | 9 | 12 | 8 | 8 | 13 | 6 | 6 |
| YEP | 36 | 32 | 7 | 8 | 5 | 5 | 21 | 10 | 7 |
| AV | 8 | 46 | 29 | 3 | 14 | 12 | 6 | 20 | 25 |
| AE | 25 | 34 | 15 | 14 | 19 | 11 | 5 | 12 | 11 |
| AEP | 22 | 26 | 27 | 15 | 12 | 7 | 13 | 13 | 12 |
| Average # GnRH Perikarya | Average # GnRH+/GPER+ Perikarya | Average # Sections | |||||||
| Rostral | Medial | Caudal | Rostral | Medial | Caudal | Rostral | Medial | Caudal | |
| YV | 2.1 | 3.5 | 0.8 | 0.8 | 2.4 | 1.8 | 2.3 | 1.4 | 1.6 |
| YE | 2.3 | 3.0 | 1.6 | 0.9 | 1.2 | 1.5 | 2.4 | 1.2 | 1.4 |
| YEP | 2.1 | 3.0 | 1.5 | 1.5 | 0.8 | 2.5 | 4.2 | 2.0 | 1.4 |
| AV | 1.2 | 2.1 | 1.2 | 1.0 | 1.2 | 2.0 | 0.8 | 2.5 | 3.3 |
| AE | 6.4 | 3.1 | 1.4 | 2.7 | 2.0 | 1.5 | 0.8 | 2.3 | 1.8 |
| AEP | 1.8 | 1.8 | 1.9 | 2.2 | 1.5 | 1.1 | 1.8 | 1.8 | 1.8 |
Acknowledgments
We are grateful to Dr. Jeffrey Roberts and Mary Roberts at the California National Primate Research Center UC-Davis Primate Center for expert care of the animals. Dr. John H. Morrison and William Janssen are acknowledged for monkey work and perfusions. Dr. Weiling Yin and Megan Noel assisted with preparation and sectioning of hypothalamic tissues. Julie Hayes' expert advice with the confocal microscope was essential for the success of this project. Dr. Ed Filardo and Henryk Urbanski provided much-needed antibodies for GPER and GnRH, respectively.
Grant Support: NIH PO1 AG16765
REFERENCES
- 1.Avis NE, Brambilla D, McKinlay SM, Vass K. A longitudinal analysis of the association between menopause and depression: Results from the Massachusetts Women’s Health Study. Ann Epidemiol. 1994;4:214–220. doi: 10.1016/1047-2797(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 2.Avis NE, Brockwell S, Randolph JF, Jr, Shen S, Cain VS, Ory M, Greendale GA. Longitudinal changes in sexual functioning as women transition through menopause: Results from the Study of Women’s Health Across the Nation. Menopause. 2009;16:442–452. doi: 10.1097/gme.0b013e3181948dd0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall JE. Neuroendocrine physiology of the early and late menopause. Endocrinol Metab Clin North Am. 2004;33:637–659. doi: 10.1016/j.ecl.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 4.McEwen BS. Estrogen actions throughout the brain. Recent Prog Horm Res. 2002;57:357–384. doi: 10.1210/rp.57.1.357. [DOI] [PubMed] [Google Scholar]
- 5.Cauley JA, Robbins J, Chen Z, Cummings SR, Jackson RD, LaCroix AZ, LeBoff M, Lewis CE, McGowan J, Neuner J, Pettinger M, Stefanick ML, Wactawski-Wende J, Watts NB. Effects of estrogen plus progestin on risk of fracture and bone mineral density. JAMA. 2014;290:1729–1738. doi: 10.1001/jama.290.13.1729. [DOI] [PubMed] [Google Scholar]
- 6.Maki PM. Critical window hypothesis of hormone therapy and cognition: a scientific update on clinical studies. Menopause. 2013;20:695–709. doi: 10.1097/GME.0b013e3182960cf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim JK, Alley D, Hu P, Karlamangla A, Seeman T, Crimmins EM. Changes in postmenopausal hormone therapy use since 1988. Womens Health Issues. 2007;17:338–341. doi: 10.1016/j.whi.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panay N, Fenton A. The WHI: have our worst fears come true? Climacteric. 2013;16:507–508. doi: 10.3109/13697137.2013.836789. [DOI] [PubMed] [Google Scholar]
- 9.Yoon B, Nyirjesy I, Billingsley FS. WHIMS trial: Letter to the editors. JAMA. 2003:290. [Google Scholar]
- 10.Gass M. Highlights from the latest WHI publications and the latest North American Menopause Society position statement on use of menopausal hormone therapy. Cleve Clin J Med. 2008;75(Suppl 4):S13–S16. doi: 10.3949/ccjm.75.suppl_4.s13. [DOI] [PubMed] [Google Scholar]
- 11.Lobo RA. Where are we 10 years after the Women’s Health Initiative? J Clin Endocrinol Metab. 2013;98:1771–1780. doi: 10.1210/jc.2012-4070. [DOI] [PubMed] [Google Scholar]
- 12.Gore AC. Gonadotropin-releasing hormone (GnRH) neurons: gene expression and neuroanatomical studies. Prog Brain Res. 2002;141:193–208. doi: 10.1016/S0079-6123(02)41094-1. [DOI] [PubMed] [Google Scholar]
- 13.King JC, Anthony EL. LHRH neurons and their projections in humans and other mammals: species comparisons. Peptides. 1984;5:195–207. doi: 10.1016/0196-9781(84)90277-8. [DOI] [PubMed] [Google Scholar]
- 14.Rangaraju NS, Xu JF, Harris RB. Pro-gonadotropin-releasing hormone protein is processed within hypothalamic neurosecretory granules. Neuroendocrinology. 1991;53:20–28. doi: 10.1159/000125692. [DOI] [PubMed] [Google Scholar]
- 15.Richter TA, Terasawa E. Neural mechanisms underlying the pubertal increase in LHRH release in the rhesus monkey. Trends Endocrinol Metab. 2001;12:353–359. doi: 10.1016/s1043-2760(01)00442-8. [DOI] [PubMed] [Google Scholar]
- 16.Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci. 2004;7:1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan KA, Witkin JW, Ferin M, Silverman AJ. Gonadotropin-releasing hormone neurons in the rhesus macaque are not immunoreactive for the estrogen receptor. Brain Res. 1995;685:198–200. doi: 10.1016/0006-8993(95)00352-q. [DOI] [PubMed] [Google Scholar]
- 18.Zhang C, Kelly MJ, Rønnekleiv OK. 17 β-estradiol rapidly increases ATP-sensitive potassium channel activity in gonadotropin-releasing hormone neurons [corrected] via a protein kinase signaling pathway. Endocrinology. 2010;151:4477–4484. doi: 10.1210/en.2010-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun J, Chu Z, Moenter SM. Diurnal in vivo and rapid in vitro effects of estradiol on voltage-gated calcium channels in gonadotropin-releasing hormone neurons. J Neurosci. 2010;30:3912–3923. doi: 10.1523/JNEUROSCI.6256-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abe H, Keen KL, Terasawa E. Rapid action of estrogens on intracellular calcium oscillations in primate luteinizing hormone-releasing hormone-1 neurons. Endocrinology. 2008;149:1155–1162. doi: 10.1210/en.2007-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kenealy BP, Keen KL, Terasawa E. Rapid action of estradiol in primate GnRH neurons: the role of estrogen receptor alpha and estrogen receptor beta. Steroids. 2011;76:861–866. doi: 10.1016/j.steroids.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kenealy BP, Keen KL, Rønnekleiv OK, Terasawa E. STX a novel nonsteroidal estrogenic compound, induces rapid action in primate GnRH neuronal calcium dynamics and peptide release. Endocrinology. 2011 Aug 31;152:3182–3191. doi: 10.1210/en.2011-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noel SD, Keen KL, Baumann DI, Filardo EJ, Terasawa E. Involvement of G protein-coupled receptor 30 (GPR30) in rapid action of estrogen in primate LHRH neurons. Mol Endocrinol. 2009;23:349–359. doi: 10.1210/me.2008-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abe H, Terasawa E. Firing pattern and rapid modulation of activity by estrogen in primate luteinizing hormone releasing hormone-1 neurons. Endocrinology. 2005;146:4312–4320. doi: 10.1210/en.2005-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abrahám IM, Han SK, Todman MG, Korach KS, Herbison AE. Estrogen receptor beta mediates rapid estrogen actions on gonadotropin-releasing hormone neurons in vivo. J Neurosci. 2003;23:5771–5777. doi: 10.1523/JNEUROSCI.23-13-05771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hrabovszky E, Kalló I, Szlávik N, Keller E, Merchenthaler I, Liposits Z. Gonadotropin-releasing hormone neurons express estrogen receptor-beta. J Clin Endocrinol Metab. 2007;92:2827–2830. doi: 10.1210/jc.2006-2819. [DOI] [PubMed] [Google Scholar]
- 27.Jacobi JS, Martin C, Nava G, Jeziorski MC, Clapp C, Martinez de al Escalera G. 17-Beta-estradiol directly regulates the expression of adrenergic receptors and kisspeptin/GPR54 system in GT1-7 GnRH neurons. Neuroendocrinology. 2007;86:260–269. doi: 10.1159/000107770. [DOI] [PubMed] [Google Scholar]
- 28.Naugle MM, Nguyen LT, Merceron TK, Filardo E, Janssen WG, Morrison JH, Rapp PR, Gore AC. G-protein coupled estrogen receptor, estrogen receptor α, and progesterone receptor immunohistochemistry in the hypothalamus of aging female rhesus macaques given long-term estradiol treatment. J Exp Zool A Ecol Genet Physiol. 2014;321:399–414. doi: 10.1002/jez.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gore AC, Windsor-Engnell BM, Terasawa E. Menopausal increases in pulsatile gonadotropin-releasing hormone release in a nonhuman primate (Macaca mulatta) Endocrinology. 2004;145:4653–4659. doi: 10.1210/en.2004-0379. [DOI] [PubMed] [Google Scholar]
- 30.Gilardi KV, Shideler SE, Valverde CR, Roberts JA, Lasley BL. Characterization of the onset of menopause in the rhesus macaque. Biol Reprod. 1997;57:335–340. doi: 10.1095/biolreprod57.2.335. [DOI] [PubMed] [Google Scholar]
- 31.Bachevalier J, Landis LS, Walker LC, Brickson M, Mishkin M, Price DL, Cork LC. Aged monkeys exhibit behavioral deficits indicative of widespread cerebral dysfunction. Neurobiol Aging. 1991;12:99–111. doi: 10.1016/0197-4580(91)90048-o. [DOI] [PubMed] [Google Scholar]
- 32.Hao J, Janssen WG, Tang Y, Roberts JA, McKay H, Lasley B, Allen PB, Greengard P, Rapp PR, Kordower JH, Hof PR, Morrison JH. Estrogen increases the number of spinophilin-immunoreactive spines in the hippocampus of young and aged female rhesus monkeys. J Comp Neurol. 2003;465:540–550. doi: 10.1002/cne.10837. [DOI] [PubMed] [Google Scholar]
- 33.Hao J, Rapp PR, Leffler AE, Leffler SR, Janssen WG, Lou W, McKay H, Roberts JA, Wearne SL, Hof PR, Morrison JH. Estrogen alters spine number and morphology in prefrontal cortex of aged female rhesus monkeys. J Neurosci. 2006;26:2571–2578. doi: 10.1523/JNEUROSCI.3440-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hao J, Rapp PR, Janssen WG, Lou W, Lasley BL, Hof PR, Morrison JH. Interactive effects of age and estrogen on cognition and pyramidal neurons in monkey prefrontal cortex. Proc Natl Acad Sci U S A. 2007;104:11465–11470. doi: 10.1073/pnas.0704757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hara Y, Yuk F, Puri R, Janssen WG, Rapp PR, Morrison JH. Presynaptic mitochondrial morphology in monkey prefrontal cortex correlates with working memory and is improved with estrogen treatment. Proc Natl Acad Sci U S A. 2014;111:486–491. doi: 10.1073/pnas.1311310110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci. 2003;23:5708–5714. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang AC, Hara Y, Janssen GM, Rapp PR, Morrison JH. Synaptic estrogen receptor-alpha levels in prefrontal cortex in female rhesus monkeys and their correlation with cognitive performance. J Neurosci. 2010;30:12770–12776. doi: 10.1523/JNEUROSCI.3192-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baxter MG, Roberts MT, Gee NA, Lasley BL, Morrison JH, Rapp PR. Multiple clinically relevant hormone therapy regimens fail to improve cognitive function in aged ovariectomized rhesus monkeys. Neurobiol Aging. 2013;34:1882–1890. doi: 10.1016/j.neurobiolaging.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohm DT, Bloss EB, Janssen WG, Dietz KC, Wadsworth S, Lou W, Gee NA, Lasley BL, Rapp PR, Morrison JH. Clinically relevant hormone treatments fail to induce spinogenesis in prefrontal cortex of aged female rhesus monkeys. J Neurosci. 2012;32:11700–11705. doi: 10.1523/JNEUROSCI.1881-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- 41.Filardo E, Quinn J, Pang Y, Graeber C, Shaw S, Dong J, Thomas P. Activation of the novel estrogen receptor G protein-coupled receptor 30 (GPR30) at the plasma membrane. Endocrinology. 2007;148:3236–3245. doi: 10.1210/en.2006-1605. [DOI] [PubMed] [Google Scholar]
- 42.Hammond R, Nelson D, Gibbs RB. GPR30 co-localizes with cholinergic neurons in the basal forebrain and enhances potassium-stimulated acetylcholine release in the hippocampus. Psychoneuroendocrinology. 2011;36:182–192. doi: 10.1016/j.psyneuen.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kermath BA, Riha PD, Sajjad A, Gore AC. Effects of chronic NMDA-NR2b inhibition in the median eminence of the reproductive senescent female rat. J Neuroendocrinol. 2013;25:887–897. doi: 10.1111/jne.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller BH, Gore AC. N-Methyl-D-aspartate receptor subunit expression in GnRH neurons changes during reproductive senescence in the female rat. Endocrinology. 2002;143:3568–3574. doi: 10.1210/en.2002-220346. [DOI] [PubMed] [Google Scholar]
- 45.Urbanski HF. Monoclonal antibodies to luteinizing hormone-releasing hormone: production, characterization, and immunocytochemical application. Biol Reprod. 1991;44:681–686. doi: 10.1095/biolreprod44.4.681. [DOI] [PubMed] [Google Scholar]
- 46.Witkin JW. Luteinizing hormone releasing hormone (LHRH) neurons in aging female rhesus macaques. Neurobiol Aging. 1986;7:259–263. doi: 10.1016/0197-4580(86)90005-9. [DOI] [PubMed] [Google Scholar]
- 47.Goldsmith PC, Song T. The gonadotropin-releasing hormone containing ventral hypothalamic tract in the fetal rhesus monkey (Macaca mulatta) J Comp Neurol. 1987;257:130–139. doi: 10.1002/cne.902570110. [DOI] [PubMed] [Google Scholar]
- 48.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kruskal W, Wallis W. Use of ranks in one-criterion variance analysis. JASA. 1952;47:582–621. [Google Scholar]
- 50.Wilcoxon F. Individual comparisons by ranking methods. Biometrics. 1945;1:80–83. [Google Scholar]
- 51.Brailoiu E, Dun SL, Brailoiu GC, Mizuo K, Sklar LA, Oprea TI, Prossnitz ER, Dun NJ. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J Endocrinol. 2007;193:311–321. doi: 10.1677/JOE-07-0017. [DOI] [PubMed] [Google Scholar]
- 52.Canonaco M, Giusi G, Madeo A, Facciolo RM, Lappano R, Canonaco A, Maggiolini M. A sexually dimorphic distribution pattern of the novel estrogen receptor G-protein-coupled receptor 30 in some brain areas of the hamster. J Endocrinol. 2008;196:131–138. doi: 10.1677/JOE-07-0392. [DOI] [PubMed] [Google Scholar]
- 53.Hazell GGJ, Yao ST, Roper JA, Prossnitz ER, O’Carroll AM, Lolait SJ. Localisation of GPR30, a novel G protein-coupled oestrogen receptor, suggests multiple functions in rodent brain and peripheral tissues. J Endocrinol. 2009;202:223–236. doi: 10.1677/JOE-09-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walker DM, Kirson D, Perez LF, Gore AC. Molecular profiling of postnatal development of the hypothalamus in female and male rats. Biol Reprod. 2012;87:129. doi: 10.1095/biolreprod.112.102798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Romanò N, Lee K, Abrahám IM, Jasoni CL, Herbison AE. Nonclassical estrogen modulation of presynaptic GABA terminals modulates calcium dynamics in gonadotropin-releasing hormone neurons. Endocrinology. 2008;149:5335–5344. doi: 10.1210/en.2008-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chu Z, Andrade J, Shupnik MA, Moenter SM. Differential regulation of gonadotropin-releasing hormone neuron activity and membrane properties by acutely applied estradiol: dependence on dose and estrogen receptor subtype. J Neurosci. 2009;29:5616–5627. doi: 10.1523/JNEUROSCI.0352-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cheong RY, Porteous R, Chambon P, Abrahám IM, Herbison AE. Effects of neuron-specific estrogen receptor (ER) α and ERβ deletion on the acute estrogen negative feedback mechanism in adult female mice. Endocrinology. 2014;155:1418–1427. doi: 10.1210/en.2013-1943. [DOI] [PubMed] [Google Scholar]
- 58.Terasawa E, Kenealy BP. Neuroestrogen, rapid action of estradiol, and GnRH neurons. Front Neuroendocrinol. 2012;33:364–375. doi: 10.1016/j.yfrne.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meyer MR, Amann K, Field AS, Hu C, Hathaway HJ, Kanagy NL, Walker MK, Barton M, Prossnitz ER. Deletion of G protein-coupled estrogen receptor increases endothelial vasoconstriction. Hypertension. 2012;59:507–512. doi: 10.1161/HYPERTENSIONAHA.111.184606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol. 2011;7:715–726. doi: 10.1038/nrendo.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Christian CA, Moenter SM. The neurobiology of preovulatory and estradiol-induced gonadotropin-releasing hormone surges. Endocr Rev. 2010;31:544–577. doi: 10.1210/er.2009-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Almey A, Filardo EJ, Milner TA, Brake WG. Estrogen receptors are found in glia and at extranuclear neuronal sites in the dorsal striatum of female rats: evidence for cholinergic but not dopaminergic colocalization. Endocrinology. 2012;153:5373–5383. doi: 10.1210/en.2012-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Isensee J, Meoli L, Zazzu V, Nabzdyk C, Witt H, Soewarto D, Effertz K, Fuchs H, Gailus-Durner V, Busch D, Adler T, Angelis MH, Irgang M, Otto C, Noppinger PR. Expression pattern of G protein-coupled receptor 30 in LacZ reporter mice. Endocrinology. 2009;150:1722–1730. doi: 10.1210/en.2008-1488. [DOI] [PubMed] [Google Scholar]
- 64.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 65.Cheng SB, Graeber CT, Quinn JA, Filardo EJ. Retrograde transport of the transmembrane estrogen receptor, G-protein-coupled-receptor-30 (GPR30/GPER) from the plasma membrane towards the nucleus. Steroids. 2011;76:892–896. doi: 10.1016/j.steroids.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 66.Rubin BS, King JC. The number and distribution of detectable luteinizing hormone (LH)-releasing hormone cell bodies changes in association with the preovulatory LH surge in the brains of young but not middle-aged female rats. Endocrinology. 1994;134:467–474. doi: 10.1210/endo.134.1.8275960. [DOI] [PubMed] [Google Scholar]
- 67.Hoffman GE, Finch CE. LHRH neurons in the female C57BL/6J mouse brain during reproductive aging: no loss up to middle age. Neurobiol Aging. 1986;7:45–48. doi: 10.1016/0197-4580(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 68.Rubin BS, King JC, Bridges RS. Immunoreactive forms of luteinizing hormone-releasing hormone in the brains of aging rats exhibiting persistent vaginal estrus. Biol Reprod. 1984;31:343–351. doi: 10.1095/biolreprod31.2.343. [DOI] [PubMed] [Google Scholar]
- 69.Funabashi T, Kimura F. The number of luteinizing hormone-releasing hormone immunoreactive neurons is significantly decreased in the forebrain of old-aged female rats. Neurosci Lett. 1995;189:85–88. doi: 10.1016/0304-3940(95)11457-8. [DOI] [PubMed] [Google Scholar]
- 70.King JC, Rubin BS. Dynamic alterations in luteinizing hormone-releasing hormone (LHRH) neuronal cell bodies and terminals of adult rats. Cell Mol Neurobiol. 1995;15:89–106. doi: 10.1007/BF02069560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yin W, Wu D, Noel ML, Gore AC. Gonadotropin-releasing hormone neuroterminals and their microenvironment in the median eminence: effects of aging and estradiol treatment. Endocrinology. 2009;150:5498–5508. doi: 10.1210/en.2009-0679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ojeda SR, Lomniczi a, Sandau US. Glial-gonadotrophin hormone (GnRH) neurone interactions in the median eminence and the control of GnRH secretion. J Neuroendocrinol. 2008;20:732–742. doi: 10.1111/j.1365-2826.2008.01712.x. [DOI] [PubMed] [Google Scholar]
- 73.Rodríguez EM, Blázquez JL, Guerra M. The design of barriers in the hypothalamus allows the median eminence and the arcuate nucleus to enjoy private milieus: the former opens to the portal blood and the latter to the cerebrospinal fluid. Peptides. 2010;31:757–776. doi: 10.1016/j.peptides.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 74.Yin W, Gore AC. The hypothalamic median eminence and its role in reproductive aging. Ann N Y Acad Sci. 2010;1204:113–122. doi: 10.1111/j.1749-6632.2010.05518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rubin BS, Bridges RS. Alterations in luteinizing hormone-releasing hormone release from the mediobasal hypothalamus of ovariectomized, steroid-primed middle-aged rats as measured by push-pull perfusion. Neuroendocrinology. 1989;49:225–232. doi: 10.1159/000125121. [DOI] [PubMed] [Google Scholar]
- 76.King JC, Letourneau RJ. Luteinizing hormone-releasing hormone terminals in the median eminence of rats undergo dramatic changes after gonadectomy, as revealed by electron microscopic image analysis. Endocrinology. 1994;134:1340–1351. doi: 10.1210/endo.134.3.8119174. [DOI] [PubMed] [Google Scholar]
- 77.Yin W, Mendenhall JM, Monita M, Gore AC. Three-dimensional properties of GnRH neuroterminals in the median eminence of young and old rats. J Comp Neurol. 2009;517:284–295. doi: 10.1002/cne.22156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ojeda SR, Ma YJ, Lee BJ, Prevot V. Glia-to-neuron signaling and the neuroendocrine control of female puberty. Recent Prog Horm Res. 2000;55:197–224. [PubMed] [Google Scholar]
- 79.Kermath BA, Riha PD, Woller MJ, Wolfe A, Gore AC. Hypothalamic molecular changes underlying natural reproductive senescence in the female rat. Endocrinology. 2014 doi: 10.1210/en.2014-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]