Abstract
Currently, many gastrointestinal diseases are a major reason for the increased mortality rate of children and adults every year. Additionally, these patients may cope with the high cost of the parenteral nutrition (PN), which aids in the long-term survival of the patients. Other treatment options include surgical lengthening, which is not sufficient in many cases, and intestinal transplantation. However, intestinal transplantation is still accompanied by many challenges, including immune rejection and donor availability, which may limit the transplant’s success. The development of more safe and promising alternative treatments for intestinal diseases is still ongoing. Stem cell-based therapy (SCT) and tissue engineering (TE) appear to be the next promising choices for the regeneration of the damaged intestine. However, suitable stem cell source is required for the SCT and TE process. Thus, in this review we discuss how intestinal stem cells (ISCs) are a promising cell source for small intestine diseases. We will also discuss the different markers were used to identify ISCs. Moreover, we discuss the dominant Wnt signaling pathway in the ISC niche and its involvement in some intestinal diseases. Additionally, we discuss ISC culture and expansion, which are critical to providing enough cells for SCT and TE. Finally, we conclude and recommend that ISC isolation, culture and expansion should be considered when SCT is a treatment option for intestinal disorders. Therefore, we believe that ISCs should be considered a cell source for SCT for many gastrointestinal diseases and should be highlighted in future clinical applications.
Keywords: Intestinal stem cells, Intestinal diseases, Stem cell-based therapy, Tissue engineering, Ex vivo culture
Introduction
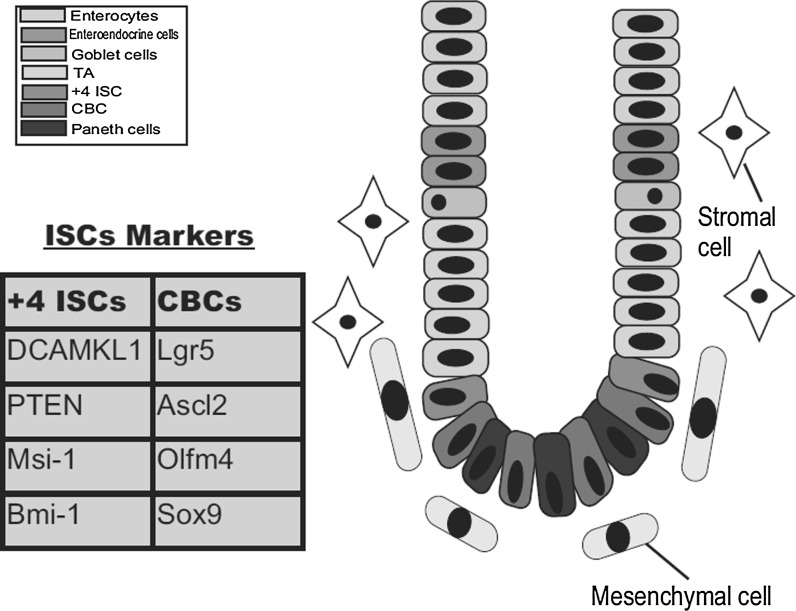
Many adult mammalian tissues possess potential stem cells that have the ability to self-renew and differentiate. One such tissue is the intestinal tissue. In anatomical terms, the intestinal tissue consists of two main parts; the small intestine and the colon (Simons and Clevers 2011; Yen and Wright 2006). Internally, this intestinal tissue is lined with epithelium. The small intestine internal layer is a mucosa that projects into the lumen in the form of long protrusions known as villi; next to these villi are the crypts of Lieberkühn. In contrast, colonic mucosa lacks the extended villi. The intestinal epithelium is the most rapidly turned over tissue (Jiang and Edgar 2012; van der Flier and Clevers 2009). Within the crypt-villus axis, we can distinguish four main cell types; the absorptive enterocytes, mucin-secreting goblet cells, enteroendocrine cells and Paneth cells (Simons and Clevers 2011). The potential stem cells of the small intestine are thought to be located in the crypt base (Shaker and Rubin 2010) as we will discuss in more detail. Intestinal stem cells (ISCs) have the ability to self-renew and can differentiate into transit amplifying progenitors (TA), which in turn give rise to the different mature epithelial cells (Fig. 1) (Montgomery and Breault 2008). Ongoing research efforts have described many ISC biomarkers, including Msi-1, Ascl2, Bmi-1, Doublecortin and Ca2+/calmodulin-dependent kinase-like 1 (DCAMKL1), and Leucin-rich repeat-containing G-protein-coupled receptor 5 (Lgr5) among others. According to previous reports, there are two ISC populations in the crypt; the quiescent cells at the +4 position above the Paneth cells and the cycling cells at the crypt bottom which are located between the Paneth cells and are known as crypt base columnar cells (CBCs) (Li and Clevers 2010; Scoville et al. 2008; May et al. 2009). In this review we will discuss the potential of ISCs in stem cell therapy for the treatment of some intestinal diseases, such as short bowel syndrome (SBS). Therefore, we will first introduce the characteristics, identifying biomarkers, location and the possible use of ISCs in tissue regeneration. Additionally, we will highlight the interactions between the ISCs and the Wnt signaling pathway and discuss their involvement in some bowel diseases. Moreover, we will discuss the possible treatments, including the stem cell-based therapy (SCT), intestinal tissue engineering (TE), and other promising therapies to recover the damaged intestinal tissue. Thus, we recommend that ISC isolation, culture and expansion are important issues that should be considered during SCT and intestinal TE.
Fig. 1.
Schematic of the crypt-villus axis in the small intestine. The diagram shows the stem cell position at the crypt base and the different mature epithelial cells including enterocytes, goblet cells, enteroendocrine cells and Paneth cells. The stem cell niche contains both mesenchymal and stromal cells. Two ISC populations reside at the crypt base and are identified by different markers
ISC location and number
Under normal conditions, every crypt has approximately 250 cells. Therefore, the crypt contains around four to six stem cells, which are responsible for daily cell compensation and renewal (Potten et al. 2002). According to previous reports, ISCs are localized at the crypt base. There are two possible hypotheses concerning the exact position of the stem cells. The first hypothesis proposes that the stem cells reside at the +4 position above the Paneth cells in the bottom of the crypt (Barker et al. 2007). These cells are DNA label-retaining cells (LRCs) , as previously reported (Sureban et al. 2011; Potten et al. 1974), and these cells have been reported to exhibit radiosensitivity. Additionally, the +4 position ISCs are a quiescent population (Potten et al. 2002). The second hypothesis proposes that the putative stem cells reside at the crypt base between the Paneth cells (Barker et al. 2007). These cells are known as the CBCs and represent the active cycling stem cells of the small intestine.
ISCs identification
DNA label retention
DNA label retention is an established method used to mark stem cells and their position in their environment. This method is based on the slow cycling and quiescent properties of stem cells. LRCs can retain a DNA synthesis label following a cellular injury (Chan and Gargett 2006). This label is then followed by a long chase period with no labeling; this chase period is long enough for the TA cells to migrate upward the villi and dilute out the DNA labeling. Additionally, ISCs have a longer cell cycle the than rapidly dividing TA cells. Thus, the ISCs retain the label, whereas the other cells do not (Barker et al. 2008). LRCs have been determined to be stem cells in the epidermal, breast, kidney and prostate tissues (Morris and Potten 1999; Bickenbach and Chism 1998; Albert et al. 2001). Using this method, identified ISCs in the intestinal crypts at positions 4–9 (Potten et al. 2002). However, the mechanisms underlying method are still unclear, and its current use for ISC identification is limited. Additional ISC identification markers have been reported and are discussed below.
ISC isolation by side population
Side population (SP) is a flow cytometry methodology used for the identification, and enrichment of stem cells in a variety of different tissues from different species. Using the Hoechst side population (SP) method to isolate cells was originally established to isolate hematopoietic stem cells (HSCs) from mouse, but it has also been used for other species and tissues. A recent study provided a protocol for the detection of putative cancer stem cells with the SP phenotype in human adherent breast cancer cell cultures (Christgen et al. 2012). Furthermore, a valuable study described the isolation of viable ISCs as a SP by fluorescence-activated cell sorting methodology (Dekaney et al. 2005).
ISCs markers
The Musashi-1 (Msi-1)
Musashi-1 (Msi-1) gene encodes an RNA binding protein that is found in neural stem cells. Msi-1 has been reported to be a regulator of Notch signaling, and its expression has been observed in the murine intestine. Although ISCs express Msi-1, mice lacking this protein do not exhibit any deficiency in crypt growth (Potten et al. 2003). Thus, Msi-1 should be investigated further before it is considered a putative ISCs marker.
DCAMKL1
DCAMKL1 or doublecortin and Ca2+/calmodulin-dependent kinase-like 1, is a microtubule-associated kinase that contains N-terminal doublecortin domains, which regulate microtubule polymerization, a C-terminal serine/threonine protein kinase domain, which is homologous a Ca2+/calmodulin-dependent kinase, and a serine/proline-rich domain located between the doublecortin and protein kinase domains, which regulates protein–protein interactions. DCAMKL1 is involved in neuronal migration and has been reported as an ISC marker that can mark quiescent stem cells. A previous study showed that cells expressing DCAMKL1 could be isolated using FACS, which demonstrates that this protein is a cell surface protein and a promising ISC marker (Sureban et al. 2011).
Bmi-1
Bmi-1 is a member of the PRC1 group (polycomb-group repressive complex 1) and is a polycomb-ring finger oncogene that is necessary for the self-renewal of hematopoietic cells and neural stem cells (Sangiorgi and Capecchi 2008). Bmi-1 null mice are deficient in hematopoietic cells and neural stem cells (Pietersen et al. 2008). Recently, Bmi-1 has been proposed as an ISC marker, as its expression has been observed at the crypt bottoms of the small intestine. Moreover, these findings were confirmed by the induction of a stable form of β-catenin in these stem cells, which eventually resulted in the formation of adenomas. Additionally, this study used Rosa26 (mutation) to inactivate Bmi-1, which resulted in crypt loss. Thus, these observations suggest that Bmi-1 can be used as a powerful ISC marker (Sangiorgi and Capecchi 2008).
Lgr5 (Gpr49)
Lgr5 or leucine-rich repeat-containing G-protein-coupled receptor 5 is a Wnt target gene. Lgr5 is a seven-transmembrane receptor and its expression has been reported in the hair follicles of mice (Uchida et al. 2010). Moreover, Lgr5+ stem cells contribute to S-shape body formation during kidney development (Barker et al. 2012). Additionally, its expression has been observed in mammary epithelial cells (Plaks et al. 2013). A previous study also showed that Lgr5 knockout mice experienced problems in their tongues and lower jaws (Haegebarth and Clevers 2009). Recently, Barker et al. (2007) introduced Lgr5 as an ISC marker gene and they performed in situ hybridization of the small intestine and showed that Lgr5 was strongly expressed in the CBCs at the crypt base. Lgr5 cells in the crypt base are cycling and rapidly dividing cells, as determined by their positive ki67 staining, which are located between the Paneth cells (Barker et al. 2007). This study also revealed that the cycling time of the CBCs was almost 24 h. A deep study of Lgr5 expression in the CBCs using knock-in alleles and a lac-z reporter confirmed their results not only in the small intestine, but also in the colon and stomach (Haegebarth and Clevers 2009). A recent study demonstrated that Lgr5 null mice exhibited premature differentiation of the Paneth cells and increased activation of the Wnt signaling pathway (Garcia et al. 2009). We think Lgr5 is the most suitable marker to represent ISCs. According to our experience and our recent publication, the experimental results showed that ISC number is quite correlated to the Lgr5 gene expression by the analysis of in situ hybridization (Chen et al. 2014).
Other markers
Other ISC markers have also been determined. One of these markers is mouse telomerase reverse transcriptase (mTert), which was reported as a marker of slowly cycling ISCs along the crypt-villus axis above the Paneth cells, similar to the LRCs (Montgomery et al. 2011). Cells expressing mTert can differentiate into all intestinal epithelial lineages. In addition to Lgr5, Ascl2 and Olfm4 have been proposed as markers for cycling CBCs (Kayahara et al. 2003; van der Flier et al. 2009). Phosphatase and tensin homolog (PTEN) was shown to be an ISC marker in a relationship with BMP signaling (Bone morphogenetic proteins). Moreover, epherin receptors (Ephs) mediate cell–cell communications and were reported to be ISC markers expressed at the crypt base (Chen et al. 2014). Recently, CD24 and CD44 could be used to FACS-isolate human intestinal epithelial cell populations with characteristics of active and facultative stem cells (Gracz et al. 2013). A similar study introduced CD24 as a membrane marker used for the isolation of mouse ISC fraction with the characteristics of stemness. Determination of CD24 as ISC marker was achieved by comparison of microarray data from the intestinal SP with similar data from a putative ISC fraction generated by laser capture microdissection (LCM), then CD24 was chosen as the most promising one (von Furstenberg et al. 2011). In this review we collected some of the previously used primers for the mRNA expression analysis by the quantitative real-time polymerase chain reaction (Table 1).
Table 1.
Used primers for the mRNA expression of ISCs by qRT-PCR analysis
| Marker gene | Forward sequence | Reverse sequence | Species | References |
|---|---|---|---|---|
| Msi-1 | -GGTCGCTTTTATTTATTTTTGGAT- | -CCGGAGGAGAGGGCAGAT- | Mouse | Potten et al. (2003) |
| Bmi-1 | -CACCCACAGTTCCCTCACATT- | -TCGAGGTCTACTGGCAAAGGA- | Mouse | Chen et al. (2014) |
| Lgr5 | -GAGTCAACCCAAGCCTTAGTATCC- | -CATGGGACAAATGCAACTGAAG- | Mouse | Chen et al. (2014) |
| Lgr5 | -TCTCAGCCATGGTGAACAA- | -TAGCGAATCACCAGGAAGGT- | Human | Uchida et al. (2010) |
| EphB3 | -GTAGGGTCAGGTGGGGATAAG- | -GACAGCACCAAGGGTAGGCAG- | Mouse | Chen et al. (2014) |
| Ascl2 | -AAGCACACCTTGACTGGTACG- | -AAGTGGACGTTTGCACCTTCA- | Mouse | Garcia et al. (2009) |
| mTert | -GCAGGTGAACAGCCTCCAGACAG- | -TCCTAACACGCTGGTCAAAGGGAAGC- | Mouse | Montgomery et al. (2011) |
ISCs and Wnt/β-catenin signaling
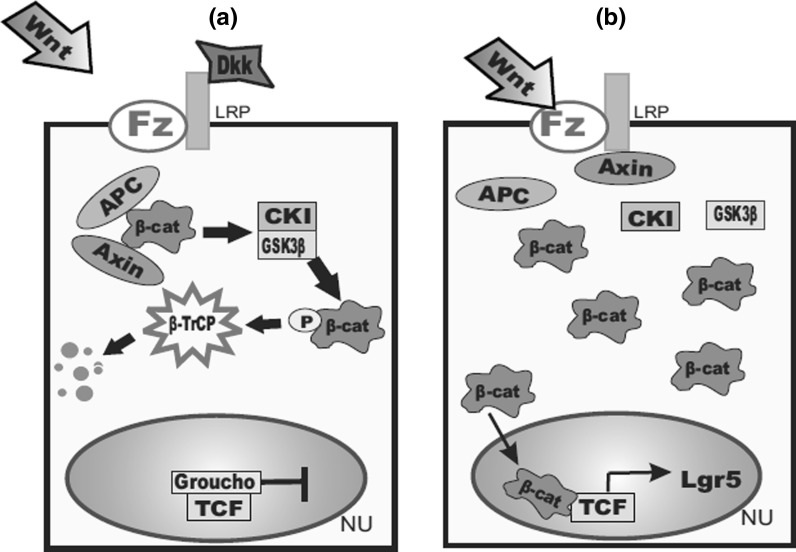
The Wnt signaling pathway is a large network of proteins that controls cell–cell communications and was originally discovered in Drosophila. The Wnt genes encode cysteine glycoproteins, which can activate cells via their interaction with the 7-transmembrane Frizzled (Fz) receptor and the single-span transmembrane protein (LRP) (Vanuytsel et al. 2013). The Wnt signaling pathway is a very complicated pathway. Wnt binds to Fz and LRP, in the canonical pathway which leads to the formation or activation of Tcf/-catenin complexes. In case of the absence of Wnt signaling, the tumor suppressor adenomatous polyposis coli (APC) and Axin displace β-catenin, allowing casein kinase I (CKI) to phosphorylate the N-terminus of β-catenin at serine S45 (Vanuytsel et al. 2013). Glycogen synthase kinase-3β (GSK3 β) can then phosphorylate additional serine and threonine residues and the phosphorylated β-catenin is recognized by transducin repeat-containing protein (TrCP), which regulates ubiquitination and degradation of β-catenin. All of the above mentioned proteins that mediated β-catenin destruction are members of the β-catenin destruction complex (Vanuytsel et al. 2013). Alternatively, Wnts can induce the phosphorylation of the cytoplasmic tail of LRP, which permits the attachment of Axin to LRP. The attachment of Axin to the membrane is thought to disrupt the destruction complex, leading to the release of β-catenin. Stabilization of β-catenin is controlled by protein phosphatase (PP2A), which dephosphorylates β-catenin. Consequently, β-catenin is translocated into the nucleus, where it associates with the Tcf transcription factors resulting in the transcription of Tcf target genes, including Lgr5 (Fig. 2) (Huelsken and Behrens 2002). The Wnt pathway can be blocked by the binding of Dkk (Wnt antagonist) to LRP.
Fig. 2.
The Canonical Wnt signaling pathway. a In the absence of Wnt stimulation, β-catenin levels are kept at a minimum through the action of the destruction complex composed of APC, Axin, GSK3 β, and CKI. In the nucleus, Tcf factors associate with transcriptional repressors, such as Groucho to block target gene activation. b In the presence of Wnt stimulation, the destruction complex is destabilized, and β-catenin accumulates in the nucleus to activate the transcription of the Tcf target genes
Wnt signaling, intestinal homeostasis and diseases’ linkage
The canonical Wnt pathway is the major force regulating the proliferation of the intestinal epithelium and controlling the balance between self-renewal and differentiation. From this standpoint, it has been observed in many studies that inhibiting the β-catenin pathway resulted in undesirable effects in the proliferation process. Previous studies showed that the upregulation of the Wnt antagonist Dkk in mice resulted in severe crypt loss, whereas its downregulation resulted in significant regeneration of the intestinal tissue (Pinto et al. 2003). Similarly, overexpression of Dkk1 resulted in the transformation of the crypt cells into mature enterocytes (Najdi et al. 2011). As a Wnt target gene, LGR5 may play a role in each stage of colorectal cancer (CRC) and be involved in cancer progression. APC, the adenomatous polyposis coli gene, is a strong negative regulator of Wnt signaling, and many reports have shown that depletion of APC in mice resulted in a significant increase in the nuclear localization of β-catenin and the increased size of the intestinal crypts. Additionally, the lack of APC results in the high expression of Wnt target genes, such as Lgr5 and c-Myc (Reya and Clevers 2005). Ephs, which were mentioned above as ISC markers, function in the cell–cell communications and have been described as Wnt target genes (van de Wetering et al. 2002; Xu et al. 1999; Sansom et al. 2004). Mice lacking EphB2 exhibited displaced TA cells; these cells were no longer found above the Paneth, but had instead moved to other positions on the crypt-villus axis (van de Wetering et al. 2002). Many studies reported that a Tcf4 knockout mouse was born with impaired crypt proliferation and with an insufficient number of villi (Logan and Nusse 2004). Many reports showed that mutations in Wnt3 resulted in the autosomal recessive human disease tetra-amelia, which is characterized by the absence of all four limbs (Gupta et al. 2006). All of these observations indicated that the Wnt pathway is very important in the crypt and stem cells maintenance. Therefore, any interruption in this pathway can result in the dysfunction of many cellular processes. Recently, intestinal tissue damage and defects have taken spotlight because of the high demand for applicable and effective therapies for some gastrointestinal diseases that lead to patient mortality during the pediatric surgery, such as inflammatory bowel disease (IBD), necrotizing enterocolitis (NEC) and SBS (Markel et al. 2008).
Short bowel syndrome (SBS)
Short bowel syndrome is an intestinal disease characterized by malabsorption due to the surgical removal of up to 70 % of the small intestine (Byrne et al. 2005). SBS symptoms include diarrhea, weight loss, fluid depletion, malnutrition and finally patient mortality. Moreover, SBS in adults and children is caused by severe surgical removal of the small intestine due to Crohn’s disease, tumors or NEC (Ekema et al. 2009). SBS treatment includes anti-diarrheal agents, vitamins and parenteral nutrition (PN) or total parenteral nutrition (TPN), which provide all the needed nutrients for the patients through an intravenous pump (Wulkersdorfer et al. 2011). However, long-term TPN can have its own complications, such as infections, thrombosis and fatty liver. Additionally, long-term TPN is costly. Another choice for the SBS treatment is sequential intestinal lengthening. This method lengthens the bowels and may help the patient to recover without the need of intestinal transplantation (Kirkman 1984), which is another alternative for the treatment of SBS and other intestinal disorders (Rocha and Whang 2004; Wada et al. 2006). However, intestinal transplantation remains one of the treatment options for patients.
Alternative treatments for intestinal diseases
Intestinal transplantation
Intestinal transplantation provides a new functional small intestine for the patients. Intestinal transplantation has evolved in the last decades as an experimental procedure to what is considered today as a long-term option for patients with intestinal failure who have developed irreversible complications associated with the chronic use of PN. However, intestinal transplantation still faces many challenges, such as immune rejection and donor availability, which limit the success of the transplant (Reyes et al. 2002; Bitar and Raghavan 2012). Based on these reasons, research is still ongoing to uncover alternative solutions for patients with SBS and other intestinal diseases.
SCT and TE
Tissue engineering is an important interdisciplinary field that involves the principles and methods of bioengineering, material science, and life sciences. The general principles of TE involve the development of valid autologous organs and tissues that can replace damaged ones with the same original function and structure. Many tissues and organs have been developed using TE, such as bone, cartilage, tendons, blood vessels, skin, liver, stomach, colon and small intestine (Langer 2000; Chen and Beierle 2004; Day 2006; Kassem et al. 2004). The development of engineered tissues requires four major components: scaffold, growth factors, extracellular matrix (ECM), and cells. Scaffolds represent important components for TE. Scaffolds, mostly made of polymeric biomaterials, provide the structural support for cell attachment and tissue development (Chan and Leong 2008). Scaffolds should provide (1) proper architecture for tissue formation and vascularization, (2) tissue compatibility, (3) should facilitate and regulate the activities of engineered tissues and (4) should provide the mechanical stability (Carletti et al. 2011). The first TE trial in the laboratory was performed by Bisceglie in the 1930s (Bisceglie 1933). Previous trials used poly-glycolic acid as suitable biodegradable scaffolds for intestinal TE (Hori et al. 2002). However, other studies have seeded canine mesenchymal stem cells (MSCs) on collagen sponge scaffold. One important factor for the successful TE is the choice of cells that are seeded onto the biodegradable scaffolds (Fukuda 2003). The choice of the cell source is a big challenge in TE because of the limited availability of autologous cell sources. Additionally, it is very important to isolate enough cells and expand them in vitro for seeding onto the biomaterial (Zakhem et al. 2012).
Cell sources for SCT and TE
Mesenchymal stem cells (MSCs)
Mesenchymal stem cells can be derived from bone marrow (BM) and can be used for the regeneration of mesenchymal tissues such as bone, muscles and adipose tissues. MSCs have the ability to differentiate into many cell types in different organs (Pittenger et al. 1999; Ferrari et al. 1998). In addition, MSCs derived from human BM have been proven that can differentiate into epithelial cells and would be a promising cell source for TE of the small intestine (Choi and Vacanti 1997). MSCs can be used for regeneration of mesenchymal tissues by combination of TE methods. Moreover, MSCs have the advantage of the secretion of immunoregulatory molecules such as vascular endothelial growth factor (VEGF), insuline-like growth factor-1 (IGF-1), hepatocyte growth factor (HGF) and epidermal growth factor (EGF). These factors play an important role in the inhibition of apoptosis, enhancement of angiogenesis, initiation of mitosis and differentiation. Thus, the risk for immunorejection and pathogen transmission appears to be low.
Intestinal stem cells (organoid units)
Intestinal organoid units (OU) are multicellular aggregates isolated from the intestine that contain both mucosal and mesenchymal elements. OUs can be used as a putative cell source for TE of the small intestine because of their ability to differentiate into all the mature epithelial cell types of normal small intestine mucosa. Some reports showed that epithelial cells require mesenchymal cells to support their growth and stem cells in the form of OUs. OUs seeded onto biodegradable scaffolds have been shown to differentiate into muscles, nervous tissues and the architecture of the small intestine, which was similar to the original tissue (Evans et al. 1992; Grikscheit et al. 2004). However, ISC transplantation may limit in autologous transplantation due to immunorejection. Previous reports have described a method to use OUs for the generation of neomucosa in dogs for the first time (Agopian et al. 2009).
Neural crest stem cells (NCSCs)
Neural crest stem cells can be isolated from gut of animals (Natarajan et al. 1999; Bondurand et al. 2003; Kruger et al. 2002; Almond et al. 2007; Metzger et al. 2009). NCSCs are a multipotent, migratory cell population unique to vertebrates that gives rise to many cell lineages including melanocytes, craniofacial cartilage and bone, smooth muscle, peripheral and enteric neurons and glia (Farlie et al. 2004; Heanue and Pachnis 2007; Nagoshi et al. 2008). During the neural crest cell migratory phase, distinctly NCSCs were found along: (1) a dorsolateral pathway, under the epidermis, as well adjacent to and intercalating through the dermamyotome; and (2) a ventral pathway, through the rostral portion of each sclerotome and around the dorsal aorta (Hall and Hörstadius 1988; Serbedzija et al. 1989, 1990). From the developmental studies, after gastrulation process, neural crest cells are specified at the border of the neural plate and the non-neural ectoderm. Besides, during neurulation, the borders of the neural plate, converge at the dorsal midline to form the neural tube.
Most neurons and glia of the enteric nervous system (ENS) originate from NCSCs (Almond et al. 2007; Belkind-Gerson et al. 2013). NCSC isolation has been reported by the means of immunoselection using specific markers and by the generation of neurospheres (Farlie et al. 2004; Kawamoto et al. 2000; Mosher et al. 2007; Lindley et al. 2008). NCSCs markers are reported such as p75 (Bixby et al. 2002), P-0 (Yamauchi et al. 1999; Kawamoto et al. 2000) and sox10 (Heanue and Pachnis 2007; Shibata et al. 2010). Msi-1 expression was also confirmed with NCSCs and NCSCs population was also derived using wnt-cre/floxp-EGFP mouse (Nagoshi et al. 2008) similar as ISCs. The first time to isolate NCSCs from mouse trunk neural tubes was to use the low affinity nerve growth factor receptor p75 marker by flow cytometry sorting (Farlie et al. 2004). These p75-positive cells mark a cell population with the ability of self-renewal and differentiation. The p75 neurotrophin receptor binds several related growth factors: nerve growth factor, brain-derived neurotrophic factor, neurotrophin-3 and neurotrophin-4. Therefore, this receptor has been demonstrated to play an important role in modulating the susceptibility of specific cell populations to programmed cell death. A recent study demonstrated the potential of the ENS as an autologous neural stem cell source. In addition, recent reports also have described that NCSCs were isolated and identified from dorsal root ganglia (DRG), BM and whisker pad (WP) in the adult transgenic mice encoding neural crest-spcecific P0-Cre/Floxed-EGFP and Wnt1-Cre/Floxed-EGFP. As a result, NCSCs from DRG had the best differentiation ability among these three cell sources (Nagoshi et al. 2008). On the other hand, NCSCs from WP may be the better cell source among these three tissues for therapeutic treatment on intestinal diseases due to convenient collection. Besides, using the appendix as a potential target opens up a new perspective that might lead to a relatively unproblematic harvest of neural stem cells. Furthermore, NCSCs have been reported that can give rise to neurons and ganglia supporting the small bowel (Mosher et al. 2007; Nagoshi et al. 2008; Natarajan et al. 1999; Shibata et al. 2010).
Tissue-engineered small intestine (TESI)
Previous reports showed that, postnatally derived human small intestine OUs could survive in tissue culture and grow into a full thickness intestine in murine hosts. All of these differentiated cell types of the intestinal mucosa, such as enterocytes, goblet cells, enteroendocrine cells and Paneth cells, were identified. Neurons, muscle cells and myofibroblasts were also observed (Grikscheit et al. 2004). Additionally, the Sala laboratory succeeded in developing TESI in a pig model using OUs (Levin et al. 2013). Furthermore, MSCs seeded on collagen scaffolds can regenerate into intestinal tissue; however, these MSCs failed to form a muscle layer as well as a mucosal layer formed by the migration of epithelial cells from the surrounding healthy intestinal mucosa (Gupta et al. 2006). Thus, OUs remain the only promising and valid cell source for TESI development. From previous studies, we can conclude that the cell source is an important factor in small intestine TE, as the cells should have the ability to differentiate into all of the cell types present in the intestine, as well as the supporting nerves and myofibroblast cells. For these reasons, the isolation and expansion of ISCs has received a great deal of attention.
ISC culture and expansion in vitro
Intestinal stem cell isolation and expansion have faced many challenges in the last few decades because of the lack of putative markers and long-term culture methods. A previous trial described a technique to isolate single cells and intact crypts in suspension from unfixed rat intestinal mucosal epithelium (Sato et al. 2009). Previously, Slorach and colleagues observed no significant proliferation of mouse OUs cultured in uncoated flasks, but a small number of OUs could adhere and proliferate (Slorach et al. 1999). Recent reports described a method of generation of the first normal human intestinal epithelial crypt-like (HIEC) cell line and villus-like primary cultures of differentiated enterocytes (PCDE) using fetal human intestines obtained at mid-gestation (Beaulieu and Ménard 2012). The ideal culture system should maintain the proliferation ability as well as the differentiation capacity of the ISCs. In 2009, Sato and his colleagues described a long-term culture system that could maintain the ISC characteristics in vitro, and they reported that they could culture intestinal crypts in a 3D culture system. According their protocols, the crypts could grow on matrigel and form crypt-villus spherical structures, known as organoids. These organoids have two domains: the villus domain and the crypt domain as shown in (Fig. 3). These crypts were grown using the commercially available advanced medium DMEM/F12 supplemented with essential growth factors. As described above, Wnt signaling is important for the ISCs maintenance; therefore, the Wnt agonist R-spondin 1 was added to the culture medium to induce crypt proliferation (Kim et al. 2005). In addition, EGF and Noggin was added to accelerate the expansion of the crypt numbers (Haramis et al. 2004). Moreover, the addition of the Rho kinase inhibitor Y-27632 was used to inhibit anoikis of the crypt cells after the isolation process (Watanabe et al. 2007). According to the Sato method, the Notch-agonistic peptide Jag 1 was also added and acted as a cell–cell Notch signaling factor (Li et al. 1998). Using this culture system, they could maintain the characteristics of the ISCs for long periods of time, approximately 8 months. The expanded crypts were shown to include the different intestinal epithelial cell types, including enterocytes, goblet cells, Paneth cells and enteroendocrine cells, as shown by confocal microscopy (Sato et al. 2009). Recently, we enhanced ISC expansion in our lab by optimizing the growth conditions of these cells (Mohamed et al. 2014).
Fig. 3.

Intestinal organoid growth in vitro. The intestinal crypts can grow in vitro into spherical structures called organoids in vitro when incubated in the described growth conditions; these organoids contain different cell types and possess ISC characteristics
Conclusions and prospects
In this review we introduced ISCs and described the different biomarkers that can be used for their identification. Additionally, we discussed the Wnt signaling pathway, which is the most dominant signaling pathway involved in intestinal homeostasis, and its effects on ISC proliferation and differentiation. From all of the previous studies, we concluded that TE is the most promising treatment for SBS and other intestinal disorders. However, TE of the small intestine requires several critical steps, including the choice of the safest biodegradable polymer and the best cell source. ISCs have the ability to differentiate into all the mature intestinal cell types. Thus, this feature could make ISCs the first choice for the SCT and TE process. We believe that the expansion of ISCs in vitro is the most critical step to provide enough cells for the procedure. In our review we recommend that future studies should focus on finding a promising cure for SBS and other intestinal diseases using SCT and TE, which includes ISC isolation, a long-term culture system, amplification of these stem cells, suitable and safe scaffolds and finally the management of the TESI development process. Therefore, we hope that these current steps may have promise for the treatment of intestinal diseases and may help in ISC basic research on ISC.
Acknowledgments
This work was supported by National Science Council, Taiwan (101-2221-E-155-044-MY3).
Conflict of interest
The authors declare no competing interests in relation to this manuscript.
Abbreviations
- PN
Parenteral nutrition
- ISCs
Intestinal stem cells
- SCT
Stem cell-based therapy
- TE
Tissue engineering
- CBCs
Crypt base columnar cells
- DCAMKL1
Doublecortin and Ca2+/calmodulin-dependent kinase-like 1
- LRCs
Label-retaining cells
- Lgr5
Leucin-rich repeat-containing G-protein-coupled receptor 5
- mTert
Mouse telomerase reverse transcriptase
- Fz
7-Transmembrane Frizzled
- LRP
Single-span transmembrane protein
- APC
Adenomatous polyposis coli
- CKI
Axin displace β-catenin allowing casein kinaseI
- GSK3 β
Glycogen synthase kinase3 β
- PP2A
Protein phosphatase
- IBD
Inflammatory bowel disease
- NEC
Necrotizing enterocolitis
- SBS
Short bowel syndrome
- MSCs
Mesenchymal stem cells
- OU
Organoid units
- TESI
Tissue-engineered small intestine
- EGF
Epidermal growth factor
- TrCP
Transducin repeat-containing protein
Footnotes
Mahmoud Shaaban Mohamed and Yun Chen have contributed equally to this work.
References
- Agopian VG, Chen DC, Avansino JR, Stelzner M. Intestinal stem cell organoid transplantation generates neomucosa in dogs. J Gastrointest Surg. 2009;13:971–982. doi: 10.1007/s11605-009-0806-x. [DOI] [PubMed] [Google Scholar]
- Albert MR, Foster RA, Vogel JC. Murine epidermal label-retaining cells isolated by flow cytometry do not express the stem cell markers CD34, Sca-1, or Flk-1. J Invest Dermatol. 2001;117:943–948. doi: 10.1046/j.0022-202x.2001.01517.x. [DOI] [PubMed] [Google Scholar]
- Almond S, Lindley RM, Kenny SE, Connell MG, Edgar DH. Characterisation and transplantation of enteric nervous system progenitor cells. Gut. 2007;56:489–496. doi: 10.1136/gut.2006.094565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Barker N, van de Wetering M, Clevers H. The intestinal stem cell. Genes Dev. 2008;22:1856–1864. doi: 10.1101/gad.1674008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, Rookmaaker MB, Kujala P, Ng A, Leushacke M, Snippert H, van de Wetering M, Tan S, Van Es JH, Huch M, Poulsom R, Verhaar MC, Peters PJ, Clevers H. Lgr5(+ve) stem/progenitor cells contribute to nephron formation during kidney development. Cell Rep. 2012;2:540–552. doi: 10.1016/j.celrep.2012.08.018. [DOI] [PubMed] [Google Scholar]
- Beaulieu JF, Ménard D. Isolation, characterization, and culture of normal human intestinal crypt and villus cells. Methods Mol Biol. 2012;806:157–173. doi: 10.1007/978-1-61779-367-7_11. [DOI] [PubMed] [Google Scholar]
- Belkind-Gerson J, Carreon-Rodriguez A, Benedict LA, Steiger C, Pieretti A, Nagy N, Dietrich J, Goldstein AM. Nestin-expressing cells in the gut give rise to enteric neurons and glial cells. Neurogastroenterol Motil. 2013;25:61–69. doi: 10.1111/nmo.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickenbach JR, Chism E. Selection and extended growth of murine epidermal stem cells in culture. Exp Cell Res. 1998;244:184–195. doi: 10.1006/excr.1998.4163. [DOI] [PubMed] [Google Scholar]
- Bisceglie V (1933) Über die antineoplastische Immunität; heterologe Einpflanzung von Tumoren in Hühner-embryonen. Ztschr Krebsforsch 40:122–140
- Bitar KN, Raghavan S. Intestinal tissue engineering: current concepts and future vision of regenerative medicine in the gut. Neurogastroenterol Motil. 2012;24:7–19. doi: 10.1111/j.1365-2982.2011.01843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bixby S, Kruger GM, Mosher JT, Joseph NM, Morrison SJ. Cell-intrinsic differences between stem cells from different regions of the peripheral nervous system regulate the generation of neural diversity. Neuron. 2002;35:643–656. doi: 10.1016/S0896-6273(02)00825-5. [DOI] [PubMed] [Google Scholar]
- Bondurand N, Natarajan D, Thapar N, Atkins C, Pachnis V. Neuron and glia generating progenitors of the mammalian enteric nervous system isolated from foetal and postnatal gut cultures. Development. 2003;130:6387–6400. doi: 10.1242/dev.00857. [DOI] [PubMed] [Google Scholar]
- Byrne TA, Wilmore DW, Iyer K, Dibaise J, Clancy K, Robinson MK, Chang P, Gertner JM, Lautz D. Growth hormone, glutamine, and an optimal diet reduces parenteral nutrition in patients with short bowel syndrome. Ann Surg. 2005;242:655–661. doi: 10.1097/01.sla.0000186479.53295.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carletti E, Motta A, Migliaresi C. Scaffolds for tissue engineering and 3D cell culture. Methods Mol Biol. 2011;695:17–39. doi: 10.1007/978-1-60761-984-0_2. [DOI] [PubMed] [Google Scholar]
- Chan RW, Gargett CE. Identification of label-retaining cells in mouse endometrium. Stem Cells. 2006;24:1529–1538. doi: 10.1634/stemcells.2005-0411. [DOI] [PubMed] [Google Scholar]
- Chan BP, Leong KW. Scaffolding in tissue engineering: general approaches and tissue-specific considerations. Eur Spine J. 2008;17:467–479. doi: 10.1007/s00586-008-0745-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MK, Beierle EA. Animal models for intestinal tissue engineering. Biomaterials. 2004;25:1675–1681. doi: 10.1016/S0142-9612(03)00517-9. [DOI] [PubMed] [Google Scholar]
- Chen Y, Lee SH, Tsai YH, Tseng SH. Ischemic preconditioning increased the intestinal stem cell activities in the intestinal crypts in mice. J Surg Res. 2014;187:85–93. doi: 10.1016/j.jss.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Choi RS, Vacanti JP. Preliminary studies of tissue-engineered intestine using isolated epithelial organoid units on tubular synthetic biodegradable scaffolds. Transplant Proc. 1997;29:848–851. doi: 10.1016/S0041-1345(96)00164-9. [DOI] [PubMed] [Google Scholar]
- Christgen M, Ballmaier M, Lehmann U, Kreipe H. Detection of putative cancer stem cells of the side population phenotype in human tumor cell cultures. Methods Mol Biol. 2012;878:201–215. doi: 10.1007/978-1-61779-854-2_13. [DOI] [PubMed] [Google Scholar]
- Day RM. Epithelial stem cells and tissue engineered intestine. Curr Stem Cell Res Ther. 2006;1:113–120. doi: 10.2174/157488806775269124. [DOI] [PubMed] [Google Scholar]
- Dekaney CM, Rodriguez JM, Graul MC, Henning SJ. Isolation and characterization of a putative intestinal stem cell fraction from mouse jejunum. Gastroenterology. 2005;129:1567–1580. doi: 10.1053/j.gastro.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Ekema G, Milianti S, Boroni G. Total parenteral nutrition in patients with short bowel syndrome. Minerva Pediatr. 2009;61:283–291. [PubMed] [Google Scholar]
- Evans GS, Flint N, Somers AS, Eyden B, Potten CS. The development of a method for the preparation of rat intestinal epithelial cell primary cultures. J Cell Sci. 1992;101:219–231. doi: 10.1242/jcs.101.1.219. [DOI] [PubMed] [Google Scholar]
- Farlie PG, McKeown SJ, Newgreen DF. The neural crest: basic biology and clinical relationships in the craniofacial and enteric nervous systems. Birth Defects Res C. 2004;72:173–189. doi: 10.1002/bdrc.20013. [DOI] [PubMed] [Google Scholar]
- Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavilio F. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- Fukuda K. Regeneration of cardiomyocytes from bone marrow: use of mesenchymal stem cell for cardiovascular tissue engineering. Cytotechnology. 2003;41:165–175. doi: 10.1023/A:1024882908173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia MI, Ghiani M, Lefort A, Libert F, Strollo S, Vassart G. LGR5 deficiency deregulates Wnt signaling and leads to precocious Paneth cell differentiation in the fetal intestine. Dev Biol. 2009;331:58–67. doi: 10.1016/j.ydbio.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Gracz AD, Fuller MK, Wang F, Li L, Stelzner M, Dunn JC, Martin MG, Magness ST. CD24 and CD44 mark human intestinal epithelial cell populations with characteristics of active and facultative stem cells. Stem Cells. 2013;31:2024–2030. doi: 10.1002/stem.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grikscheit TC, Siddique A, Ochoa ER, Srinivasan A, Alsberg E, Hodin RA, Vacanti JP. Tissue-engineered small intestine improves recovery after massive small bowel resection. Ann Surg. 2004;240:748–754. doi: 10.1097/01.sla.0000143246.07277.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Dixit A, Sales KM, Winslet MC, Seifalian AM. Tissue engineering of small intestines—current status. Biomacromolecules. 2006;7:2701–2709. doi: 10.1021/bm060383e. [DOI] [PubMed] [Google Scholar]
- Haegebarth A, Clevers H. Wnt signaling, lgr5, and stem cells in the intestine and skin. Am J Pathol. 2009;174:715–721. doi: 10.2353/ajpath.2009.080758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall BK, Hörstadius S. The neural crest. London: Oxford University Press; 1988. [Google Scholar]
- Haramis AP, Begthel H, van den Born M, van Es J, Jonkheer S, Offerhaus GJ, Clevers H. De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science. 2004;303:1684–1686. doi: 10.1126/science.1093587. [DOI] [PubMed] [Google Scholar]
- Heanue TA, Pachnis V. Enteric nervous system development and Hirschsprung’s disease: advances in genetic and stem cell studies. Nat Rev Neurosci. 2007;8:466–479. doi: 10.1038/nrn2137. [DOI] [PubMed] [Google Scholar]
- Hori Y, Nakamura T, Kimura D, Kaino K, Kurokawa Y, Satomi S, Shimizu Y. Experimental study on tissue engineering of the small intestine by mesenchymal stem cell seeding. J Surg Res. 2002;102:156–160. doi: 10.1006/jsre.2001.6294. [DOI] [PubMed] [Google Scholar]
- Huelsken J, Behrens J. The Wnt signalling pathway. J Cell Sci. 2002;115:3977–3978. doi: 10.1242/jcs.00089. [DOI] [PubMed] [Google Scholar]
- Jiang H, Edgar BA. Intestinal stem cell function in Drosophila and mice. Curr Opin Genet Dev. 2012;22:354–360. doi: 10.1016/j.gde.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassem M, Abdallah BM, Yu Z, Ditzel N, Burns JS. The use of hTERT-immortalized cells in tissue engineering. Cytotechnology. 2004;45:39–46. doi: 10.1007/s10616-004-5124-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto S, Niwa H, Tashiro F, Sano S, Kondoh G, Takeda J, Tabayashi K, Miyazaki J. A novel reporter mouse strain that expresses enhanced green fluorescent protein upon Cre-mediated recombination. FEBS Lett. 2000;470:263–268. doi: 10.1016/S0014-5793(00)01338-7. [DOI] [PubMed] [Google Scholar]
- Kayahara T, Sawada M, Takaishi S, Fukui H, Seno H, Fukuzawa H, Suzuki K, Hiai H, Kageyama R, Okano H, Chiba T. Candidate markers for stem and early progenitor cells, Musashi-1 and Hes1, are expressed in crypt base columnar cells of mouse small intestine. FEBS Lett. 2003;535:131–135. doi: 10.1016/S0014-5793(02)03896-6. [DOI] [PubMed] [Google Scholar]
- Kim KA, Kakitani M, Zhao J, Oshima T, Tang T, Binnerts M, Liu Y, Boyle B, Park E, Emtage P, Funk WD, Tomizuka K. Mitogenic influence of human R-spondin1 on the intestinal epithelium. Science. 2005;309:1256–1259. doi: 10.1126/science.1112521. [DOI] [PubMed] [Google Scholar]
- Kirkman RL. Small bowel transplantation. Transplantation. 1984;37:429–433. doi: 10.1097/00007890-198405000-00001. [DOI] [PubMed] [Google Scholar]
- Kruger GM, Mosher JT, Bixby S, Joseph N, Iwashita T, Morrison SJ. Neural crest stem cells persist in the adult gut but undergo changes in self-renewal, neuronal subtype potential, and factor responsiveness. Neuron. 2002;35:657–669. doi: 10.1016/S0896-6273(02)00827-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer R. Tissue engineering. Mol Ther. 2000;1:12–15. doi: 10.1006/mthe.1999.0003. [DOI] [PubMed] [Google Scholar]
- Levin DE, Barthel ER, Speer AL, Sala FG, Hou X, Torashima Y, Grikscheit TC. Human tissue-engineered small intestine forms from postnatal progenitor cells. J Pediatr Surg. 2013;48:129–137. doi: 10.1016/j.jpedsurg.2012.10.029. [DOI] [PubMed] [Google Scholar]
- Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327:542–545. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Milner LA, Deng Y, Iwata M, Banta A, Graf L, Marcovina S, Friedman C, Trask BJ, Hood L, Torok-Storb B. The human homolog of rat Jagged1 expressed by marrow stroma inhibits differentiation of 32D cells through interaction with Notch1. Immunity. 1998;8:43–55. doi: 10.1016/S1074-7613(00)80457-4. [DOI] [PubMed] [Google Scholar]
- Lindley RM, Hawcutt DB, Connell MG, Almond SL, Vannucchi MG, Faussone-Pellegrini MS, Edgar DH, Kenny SE. Human and mouse enteric nervous system neurosphere transplants regulate the function of aganglionic embryonic distal colon. Gastroenterology. 2008;135:205–216. doi: 10.1053/j.gastro.2008.03.035. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Markel TA, Crisostomo PR, Lahm T, Novotny NM, Rescorla FJ, Tector J, Meldrum DR. Stem cells as a potential future treatment of pediatric intestinal disorders. J Pediatr Surg. 2008;43:1953–1963. doi: 10.1016/j.jpedsurg.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May R, Sureban SM, Hoang N, Riehl TE, Lightfoot SA, Ramanujam R, Wyche JH, Anant S, Houchen CW. Doublecortin and CaM kinase-like-1 and leucine-rich-repeat-containing G-protein-coupled receptor mark quiescent and cycling intestinal stem cells, respectively. Stem Cells. 2009;27:2571–2579. doi: 10.1002/stem.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger M, Caldwell C, Barlow AJ, Burns AJ, Thapar N. Enteric nervous system stem cells derived from human gut mucosa for the treatment of aganglionic gut disorders. Gastroenterology. 2009;136:2214–2225. doi: 10.1053/j.gastro.2009.02.048. [DOI] [PubMed] [Google Scholar]
- Mohamed MS, Chen Y, Yao CL (2014) A serum-free medium developed for in vitro expansion of murine intestinal stem cells. Biotechnol J. doi:10.1002/biot.201400016 [DOI] [PubMed]
- Montgomery RK, Breault DT. Small intestinal stem cell markers. J Anat. 2008;213:52–58. doi: 10.1111/j.1469-7580.2008.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery RK, Carlone DL, Richmond CA, Farilla L, Kranendonk ME, Henderson DE, Baffour-Awuah NY, Ambruzs DM, Fogli LK, Algra S, Breault DT. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc Natl Acad Sci USA. 2011;108:179–184. doi: 10.1073/pnas.1013004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RJ, Potten CS. Highly persistent label-retaining cells in the hair follicles of mice and their fate following induction of anagen. J Invest Dermatol. 1999;112:470–475. doi: 10.1046/j.1523-1747.1999.00537.x. [DOI] [PubMed] [Google Scholar]
- Mosher JT, Yeager KJ, Kruger GM, Joseph NM, Hutchin ME, Dlugosz AA, Morrison SJ. Intrinsic differences among spatially distinct neural crest stem cells in terms of migratory properties, fate determination, and ability to colonize the enteric nervous system. Dev Biol. 2007;303:1–15. doi: 10.1016/j.ydbio.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagoshi N, Shibata S, Kubota Y, Nakamura M, Nagai Y, Satoh E, Morikawa S, Okada Y, Mabuchi Y, Katoh H, Okada S, Fukuda K, Suda T, Matsuzaki Y, Toyama Y, Okano H. Ontogeny and multipotency of neural crest-derived stem cells in mouse bone marrow, dorsal root ganglia, and whisker pad. Cell Stem Cell. 2008;2:392–403. doi: 10.1016/j.stem.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Najdi R, Holcombe RF, Waterman ML. Wnt signaling and colon carcinogenesis: beyond APC. J Carcinog. 2011;10:5. doi: 10.4103/1477-3163.78111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan D, Grigoriou M, Marcos-Gutierrez CV, Atkins C, Pachnis V. Multipotential progenitors of the mammalian enteric nervous system capable of colonising aganglionic bowel in organ culture. Development. 1999;126:157–168. doi: 10.1242/dev.126.1.157. [DOI] [PubMed] [Google Scholar]
- Pietersen AM, Evers B, Prasad AA, Tanger E, Cornelissen-Steijger P, Jonkers J, van Lohuizen M. Bmi1 regulates stem cells and proliferation and differentiation of committed cells in mammary epithelium. Curr Biol. 2008;18:1094–1099. doi: 10.1016/j.cub.2008.06.070. [DOI] [PubMed] [Google Scholar]
- Pinto D, Gregorieff A, Begthel H, Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003;17:1709–1713. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Plaks V, Brenot A, Lawson DA, Linnemann JR, Van Kappel EC, Wong KC, de Sauvage F, Klein OD, Werb Z. Lgr5-expressing cells are sufficient and necessary for postnatal mammary gland organogenesis. Cell Rep. 2013;3:70–78. doi: 10.1016/j.celrep.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten CS, Kovacs L, Hamilton E. Continuous labelling studies on mouse skin and intestine. Cell Tissue Kinet. 1974;7:271–283. doi: 10.1111/j.1365-2184.1974.tb00907.x. [DOI] [PubMed] [Google Scholar]
- Potten CS, Owen G, Booth D. Intestinal stem cells protect their genome by selective segregation of template DNA strands. J Cell Sci. 2002;115:2381–2388. doi: 10.1242/jcs.115.11.2381. [DOI] [PubMed] [Google Scholar]
- Potten CS, Booth C, Tudor GL, Booth D, Brady G, Hurley P, Ashton G, Clarke R, Sakakibara S, Okano H. Identification of a putative intestinal stem cell and early lineage marker; musashi-1. Differentiation. 2003;71:28–41. doi: 10.1046/j.1432-0436.2003.700603.x. [DOI] [PubMed] [Google Scholar]
- Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- Reyes J, Mazariegos GV, Bond GM, Green M, Dvorchik I, Kosmach-Park B, Abu-Elmagd K. Pediatric intestinal transplantation: historical notes, principles and controversies. Pediatr Transplant. 2002;6:193–207. doi: 10.1034/j.1399-3046.2002.02003.x. [DOI] [PubMed] [Google Scholar]
- Rocha FG, Whang EE. Intestinal tissue engineering: from regenerative medicine to model systems. J Surg Res. 2004;120:320–325. doi: 10.1016/j.jss.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet. 2008;40:915–920. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansom OJ, Reed KR, Hayes AJ, Ireland H, Brinkmann H, Newton IP, Batlle E, Simon-Assmann P, Clevers H, Nathke IS, Clarke AR, Winton DJ. Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes Dev. 2004;18:1385–1390. doi: 10.1101/gad.287404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- Scoville DH, Sato T, He XC, Li L. Current view: intestinal stem cells and signaling. Gastroenterology. 2008;134:849–864. doi: 10.1053/j.gastro.2008.01.079. [DOI] [PubMed] [Google Scholar]
- Serbedzija GN, Bronner-Fraser M, Fraser SE. A vital dye analysis of the timing and pathways of avian trunk neural crest cell migration. Development. 1989;106:809–816. doi: 10.1242/dev.106.4.809. [DOI] [PubMed] [Google Scholar]
- Serbedzija GN, Fraser SE, Bronner-Fraser M. Pathways of trunk neural crest cell migration in the mouse embryo as revealed by vital dye labelling. Development. 1990;108:605–612. doi: 10.1242/dev.108.4.605. [DOI] [PubMed] [Google Scholar]
- Shaker A, Rubin DC. Intestinal stem cells and epithelial-mesenchymal interactions in the crypt and stem cell niche. Transl Res. 2010;156:180–187. doi: 10.1016/j.trsl.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S, Yasuda A, Renault-Mihara F, Suyama S, Katoh H, Inoue T, Inoue YU, Nagoshi N, Sato M, Nakamura M, Akazawa C, Okano H. Sox10-Venus mice: a new tool for real-time labeling of neural crest lineage cells and oligodendrocytes. Mol Brain. 2010;3:31. doi: 10.1186/1756-6606-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons BD, Clevers H. Stem cell self-renewal in intestinal crypt. Exp Cell Res. 2011;317:2719–2724. doi: 10.1016/j.yexcr.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Slorach EM, Campbell FC, Dorin JR. A mouse model of intestinal stem cell function and regeneration. J Cell Sci. 1999;112:3029–3038. doi: 10.1242/jcs.112.18.3029. [DOI] [PubMed] [Google Scholar]
- Sureban SM, May R, Lightfoot SA, Hoskins AB, Lerner M, Brackett DJ, Postier RG, Ramanujam R, Mohammed A, Rao CV, Wyche JH, Anant S, Houchen CW. DCAMKL-1 regulates epithelial-mesenchymal transition in human pancreatic cells through a miR-200a-dependent mechanism. Cancer Res. 2011;71:2328–2338. doi: 10.1158/0008-5472.CAN-10-2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida H, Yamazaki K, Fukuma M, Yamada T, Hayashida T, Hasegawa H, Kitajima M, Kitagawa Y, Sakamoto M. Overexpression of leucine-rich repeat-containing G protein-coupled receptor 5 in colorectal cancer. Cancer Sci. 2010;101:1731–1737. doi: 10.1111/j.1349-7006.2010.01571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A, van der Horn K, Batlle E, Coudreuse D, Haramis AP, Tjon-Pon-Fong M, Moerer P, van den Born M, Soete G, Pals S, Eilers M, Medema R, Clevers H. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 2002;111:241–250. doi: 10.1016/S0092-8674(02)01014-0. [DOI] [PubMed] [Google Scholar]
- van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–260. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- van der Flier LG, Haegebarth A, Stange DE, van de Wetering M, Clevers H. OLFM4 is a robust marker for stem cells in human intestine and marks a subset of colorectal cancer cells. Gastroenterology. 2009;137:15–17. doi: 10.1053/j.gastro.2009.05.035. [DOI] [PubMed] [Google Scholar]
- Vanuytsel T, Senger S, Fasano A, Shea-Donohue T. Major signaling pathways in intestinal stem cells. Biochim Biophys Acta. 2013;1830:2410–2426. doi: 10.1016/j.bbagen.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Furstenberg RJ, Gulati AS, Baxi A, Doherty JM, Stappenbeck TS, Gracz AD, Magness ST, Henning SJ. Sorting mouse jejunal epithelial cells with CD24 yields a population with characteristics of intestinal stem cells. Am J Physiol Gastrointest Liver Physiol. 2011;300:G409–G419. doi: 10.1152/ajpgi.00453.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada M, Kato T, Hayashi Y, Selvaggi G, Mittal N, Thompson J, Gonzalez M, Nishida S, Madariaga J, Tzakis A. Intestinal transplantation for short bowel syndrome secondary to gastroschisis. J Pediatr Surg. 2006;41:1841–1845. doi: 10.1016/j.jpedsurg.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Nishikawa S, Muguruma K, Sasai Y. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- Wulkersdorfer B, Kao KK, Agopian VG, Dunn JC, Wu BM, Stelzner M. Growth factors adsorbed on polyglycolic acid mesh augment growth of bioengineered intestinal neomucosa. J Surg Res. 2011;169:169–178. doi: 10.1016/j.jss.2009.11.719. [DOI] [PubMed] [Google Scholar]
- Xu Q, Mellitzer G, Robinson V, Wilkinson DG. In vivo cell sorting in complementary segmental domains mediated by Eph receptors and ephrins. Nature. 1999;399:267–271. doi: 10.1038/20452. [DOI] [PubMed] [Google Scholar]
- Yamauchi Y, Abe K, Mantani A, Hitoshi Y, Suzuki M, Osuzu F, Kuratani S, Yamamura K. A novel transgenic technique that allows specific marking of the neural crest cell lineage in mice. Dev Biol. 1999;212:191–203. doi: 10.1006/dbio.1999.9323. [DOI] [PubMed] [Google Scholar]
- Yen TH, Wright NA. The gastrointestinal tract stem cell niche. Stem Cell Rev. 2006;2:203–212. doi: 10.1007/s12015-006-0048-1. [DOI] [PubMed] [Google Scholar]
- Zakhem E, Raghavan S, Gilmont RR, Bitar KN. Chitosan-based scaffolds for the support of smooth muscle constructs in intestinal tissue engineering. Biomaterials. 2012;33:4810–4817. doi: 10.1016/j.biomaterials.2012.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]