Abstract
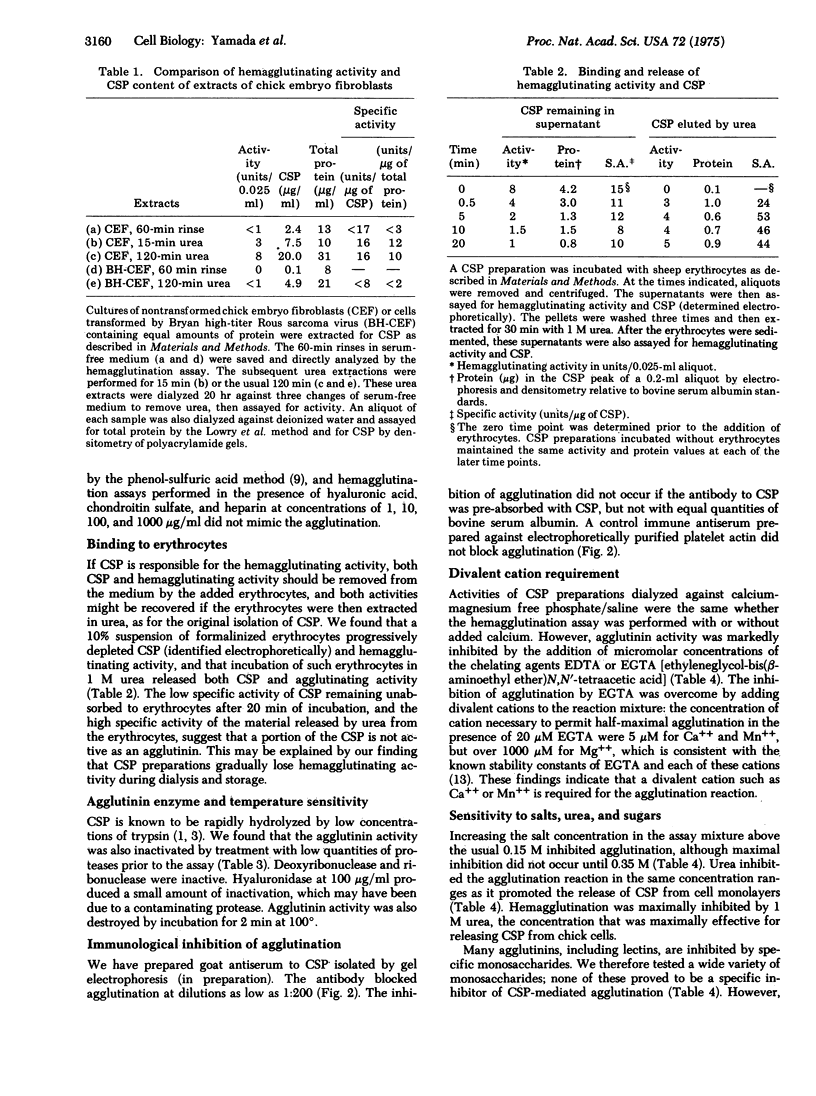
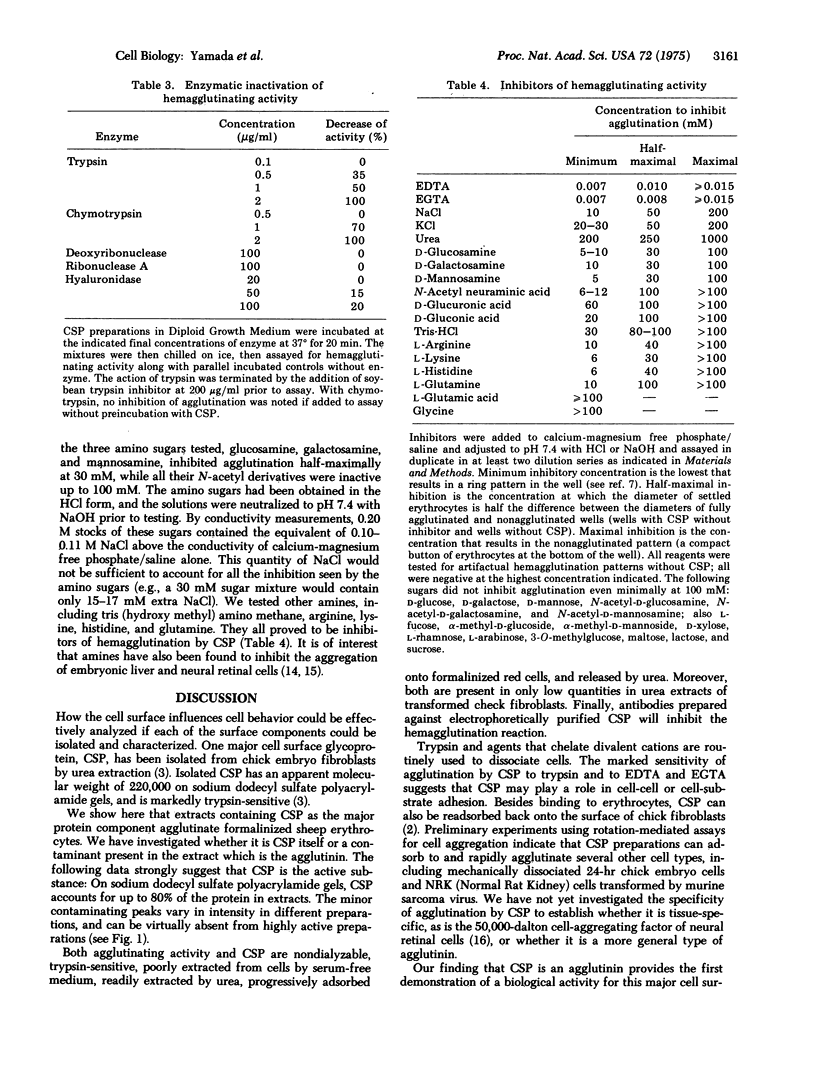
A major cell surface protein, CSP, of chick embryo fibroblasts has been shown to constitute up to 3% of total cell protein, and to be decreased after viral transformation. Its role in normal cell behavior is not known. We have isolated CSP from chick embryo fibroblasts by extraction with 1 M urea and find that these preparations of CSP agglutinate formalinized sheep erythrocytes at protein concentrations of under 2 mug/ml. In extracts of chick embryo cells, the quantity of such hemagglutinating activity parallels that of CSP determined by electrophoresis, and both are substantially decreased in chick cells transformed by the Bryan hightiter strain of Rous sarcoma virus. Both CSP and hemagglutinating activity are progressively adsorbed onto erythrocytes and can be released by 1 M urea. An antiserum to purified CSP specifically blocks the agglutination. The agglutinating activity is destroyed by boiling or treatment with proteases. The agglutination reaction is inhibited by the chelating agents EDTA and EGTA [ethyleneglycol-bis(beta-aminoethyl ether)N,N'-tetraacetic acid]. Agglutination is also inhibited to a lesser degres by amino sugars and other amines, increased osmolarity, and urea. Other monosaccharides, hyaluronidase, DNase, and RNase have little or not effect on the agglutination reaction. This demonstration that CSP has an agglutinating activity that is sensitive to proteases and that requires divalent cations suggests that this molecule may play a role in cell adhesion.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUTLER W. T. HEMAGGLUTINATION STUDIES WITH FORMALINIZED ERYTHROCYTES. EFFECT OF BIS-DIAZO-BENZIDINE AND TANNIC ACID TREATMENT ON SENSITIZATION BY SOLUBLE ANTIGEN. J Immunol. 1963 May;90:663–671. [PubMed] [Google Scholar]
- GARBER B. Inhibition by glucosamine of aggregation of dissociated embryonic cells. Dev Biol. 1963 Mar;6:630–641. doi: 10.1016/0012-1606(63)90147-7. [DOI] [PubMed] [Google Scholar]
- Glaeser R. M., Richmond J. E., Todd P. W. Histotypic self-organization by trypsin-dissociated and EDTA-dissociated chick embryo cells. Exp Cell Res. 1968 Sep;52(1):71–85. doi: 10.1016/0014-4827(68)90548-x. [DOI] [PubMed] [Google Scholar]
- Hausman R. E., Moscona A. A. Purification and characterization of the retina-specific cell-aggregating factor. Proc Natl Acad Sci U S A. 1975 Mar;72(3):916–920. doi: 10.1073/pnas.72.3.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Pessac B., Defendi V. Cell aggregation: role of acid mucopolysaccharides. Science. 1972 Feb 25;175(4024):898–900. doi: 10.1126/science.175.4024.898. [DOI] [PubMed] [Google Scholar]
- Rosen S. D., Simpson D. L., Rose J. E., Barondes S. H. Carbohydrate-binding protein from Polysphondylium pallidum implicated in intercellular adhesion. Nature. 1974 Nov 8;252(5479):128, 149-50. doi: 10.1038/252128a0. [DOI] [PubMed] [Google Scholar]
- Stone K. R., Smith R. E., Joklik W. K. Changes in membrane polypeptides that occur when chick embryo fibroblasts and NRK cells are transformed with avian sarcoma viruses. Virology. 1974 Mar;58(1):86–100. doi: 10.1016/0042-6822(74)90143-3. [DOI] [PubMed] [Google Scholar]
- Vaheri A., Ruoslahti E. Disappearance of a major cell-type specific surface glycoprotein antigen (SF) after transformation of fibroblasts by Rous sarcoma virus. Int J Cancer. 1974 May 15;13(5):579–586. doi: 10.1002/ijc.2910130502. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Weston J. A. Isolation of a major cell surface glycoprotein from fibroblasts. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3492–3496. doi: 10.1073/pnas.71.9.3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M., Weston J. A. The synthesis, turnover, and artificial restoration of a major cell surface glycoprotein. Cell. 1975 May;5(1):75–81. doi: 10.1016/0092-8674(75)90094-x. [DOI] [PubMed] [Google Scholar]