Abstract
Although several biochemical features of p53 have been described, their relationship to tumor suppression remains uncertain. We have compared the ability of p53-derived proteins to act as sequence-specific transcriptional (SST) activators with their ability to suppress tumor cell growth, using an improved growth-suppression assay. Both naturally occurring and in vitro derived mutations that abrogated the SST activity of p53 lost the ability to suppress tumor cell growth. Additionally, the N- and C-terminal ends of p53 were shown to be functionally replaceable with foreign transactivation and dimerization domains, respectively, with concordant preservation of both SST and tumor-suppressive properties. Only the central region of p53, conferring specific DNA binding, was required to suppress growth by such hybrid proteins. The SST activity of p53 thus appeared to be essential for the protein to function as a tumor suppressor.
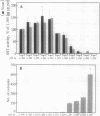
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoyama N., Nagase T., Sawazaki T., Mizuguchi G., Nakagoshi H., Fujisawa J. I., Yoshida M., Ishii S. Overlap of the p53-responsive element and cAMP-responsive element in the enhancer of human T-cell leukemia virus type I. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5403–5407. doi: 10.1073/pnas.89.12.5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker S. J., Markowitz S., Fearon E. R., Willson J. K., Vogelstein B. Suppression of human colorectal carcinoma cell growth by wild-type p53. Science. 1990 Aug 24;249(4971):912–915. doi: 10.1126/science.2144057. [DOI] [PubMed] [Google Scholar]
- Buchman A. R., Lue N. F., Kornberg R. D. Connections between transcriptional activators, silencers, and telomeres as revealed by functional analysis of a yeast DNA-binding protein. Mol Cell Biol. 1988 Dec;8(12):5086–5099. doi: 10.1128/mcb.8.12.5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. Y., Funk W. D., Wright W. E., Shay J. W., Minna J. D. Heterogeneity of transcriptional activity of mutant p53 proteins and p53 DNA target sequences. Oncogene. 1993 Aug;8(8):2159–2166. [PubMed] [Google Scholar]
- Donehower L. A., Harvey M., Slagle B. L., McArthur M. J., Montgomery C. A., Jr, Butel J. S., Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992 Mar 19;356(6366):215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- Ellenberger T. E., Brandl C. J., Struhl K., Harrison S. C. The GCN4 basic region leucine zipper binds DNA as a dimer of uninterrupted alpha helices: crystal structure of the protein-DNA complex. Cell. 1992 Dec 24;71(7):1223–1237. doi: 10.1016/s0092-8674(05)80070-4. [DOI] [PubMed] [Google Scholar]
- Fields S., Jang S. K. Presence of a potent transcription activating sequence in the p53 protein. Science. 1990 Aug 31;249(4972):1046–1049. doi: 10.1126/science.2144363. [DOI] [PubMed] [Google Scholar]
- Funk W. D., Pak D. T., Karas R. H., Wright W. E., Shay J. W. A transcriptionally active DNA-binding site for human p53 protein complexes. Mol Cell Biol. 1992 Jun;12(6):2866–2871. doi: 10.1128/mcb.12.6.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groger R. K., Morrow D. M., Tykocinski M. L. Directional antisense and sense cDNA cloning using Epstein-Barr virus episomal expression vectors. Gene. 1989 Sep 30;81(2):285–294. doi: 10.1016/0378-1119(89)90189-3. [DOI] [PubMed] [Google Scholar]
- Hu J. C., O'Shea E. K., Kim P. S., Sauer R. T. Sequence requirements for coiled-coils: analysis with lambda repressor-GCN4 leucine zipper fusions. Science. 1990 Dec 7;250(4986):1400–1403. doi: 10.1126/science.2147779. [DOI] [PubMed] [Google Scholar]
- Hupp T. R., Meek D. W., Midgley C. A., Lane D. P. Regulation of the specific DNA binding function of p53. Cell. 1992 Nov 27;71(5):875–886. doi: 10.1016/0092-8674(92)90562-q. [DOI] [PubMed] [Google Scholar]
- Jackson P., Bos E., Braithwaite A. W. Wild-type mouse p53 down-regulates transcription from different virus enhancer/promoters. Oncogene. 1993 Mar;8(3):589–597. [PubMed] [Google Scholar]
- Kern S. E., Kinzler K. W., Bruskin A., Jarosz D., Friedman P., Prives C., Vogelstein B. Identification of p53 as a sequence-specific DNA-binding protein. Science. 1991 Jun 21;252(5013):1708–1711. doi: 10.1126/science.2047879. [DOI] [PubMed] [Google Scholar]
- Kern S. E., Pietenpol J. A., Thiagalingam S., Seymour A., Kinzler K. W., Vogelstein B. Oncogenic forms of p53 inhibit p53-regulated gene expression. Science. 1992 May 8;256(5058):827–830. doi: 10.1126/science.1589764. [DOI] [PubMed] [Google Scholar]
- Mack D. H., Vartikar J., Pipas J. M., Laimins L. A. Specific repression of TATA-mediated but not initiator-mediated transcription by wild-type p53. Nature. 1993 May 20;363(6426):281–283. doi: 10.1038/363281a0. [DOI] [PubMed] [Google Scholar]
- Mercer W. E. Cell cycle regulation and the p53 tumor suppressor protein. Crit Rev Eukaryot Gene Expr. 1992;2(3):251–263. [PubMed] [Google Scholar]
- Pietenpol J. A., Vogelstein B. Tumour suppressor genes. No room at the p53 inn. Nature. 1993 Sep 2;365(6441):17–18. doi: 10.1038/365017a0. [DOI] [PubMed] [Google Scholar]
- Prives C., Manfredi J. J. The p53 tumor suppressor protein: meeting review. Genes Dev. 1993 Apr;7(4):529–534. doi: 10.1101/gad.7.4.529. [DOI] [PubMed] [Google Scholar]
- Raycroft L., Wu H. Y., Lozano G. Transcriptional activation by wild-type but not transforming mutants of the p53 anti-oncogene. Science. 1990 Aug 31;249(4972):1049–1051. doi: 10.1126/science.2144364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed M., Wang Y., Mayr G., Anderson M. E., Schwedes J. F., Tegtmeyer P. p53 domains: suppression, transformation, and transactivation. Gene Expr. 1993;3(1):95–107. [PMC free article] [PubMed] [Google Scholar]
- Stürzbecher H. W., Brain R., Addison C., Rudge K., Remm M., Grimaldi M., Keenan E., Jenkins J. R. A C-terminal alpha-helix plus basic region motif is the major structural determinant of p53 tetramerization. Oncogene. 1992 Aug;7(8):1513–1523. [PubMed] [Google Scholar]
- Subler M. A., Martin D. W., Deb S. Inhibition of viral and cellular promoters by human wild-type p53. J Virol. 1992 Aug;66(8):4757–4762. doi: 10.1128/jvi.66.8.4757-4762.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talanian R. V., McKnight C. J., Kim P. S. Sequence-specific DNA binding by a short peptide dimer. Science. 1990 Aug 17;249(4970):769–771. doi: 10.1126/science.2389142. [DOI] [PubMed] [Google Scholar]
- Triezenberg S. J., Kingsbury R. C., McKnight S. L. Functional dissection of VP16, the trans-activator of herpes simplex virus immediate early gene expression. Genes Dev. 1988 Jun;2(6):718–729. doi: 10.1101/gad.2.6.718. [DOI] [PubMed] [Google Scholar]
- Unger T., Mietz J. A., Scheffner M., Yee C. L., Howley P. M. Functional domains of wild-type and mutant p53 proteins involved in transcriptional regulation, transdominant inhibition, and transformation suppression. Mol Cell Biol. 1993 Sep;13(9):5186–5194. doi: 10.1128/mcb.13.9.5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger T., Nau M. M., Segal S., Minna J. D. p53: a transdominant regulator of transcription whose function is ablated by mutations occurring in human cancer. EMBO J. 1992 Apr;11(4):1383–1390. doi: 10.1002/j.1460-2075.1992.tb05183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B., Kinzler K. W. p53 function and dysfunction. Cell. 1992 Aug 21;70(4):523–526. doi: 10.1016/0092-8674(92)90421-8. [DOI] [PubMed] [Google Scholar]
- Zambetti G. P., Levine A. J. A comparison of the biological activities of wild-type and mutant p53. FASEB J. 1993 Jul;7(10):855–865. doi: 10.1096/fasebj.7.10.8344485. [DOI] [PubMed] [Google Scholar]
- Zhang W., Funk W. D., Wright W. E., Shay J. W., Deisseroth A. B. Novel DNA binding of p53 mutants and their role in transcriptional activation. Oncogene. 1993 Sep;8(9):2555–2559. [PubMed] [Google Scholar]
- el-Deiry W. S., Kern S. E., Pietenpol J. A., Kinzler K. W., Vogelstein B. Definition of a consensus binding site for p53. Nat Genet. 1992 Apr;1(1):45–49. doi: 10.1038/ng0492-45. [DOI] [PubMed] [Google Scholar]
- el-Deiry W. S., Tokino T., Velculescu V. E., Levy D. B., Parsons R., Trent J. M., Lin D., Mercer W. E., Kinzler K. W., Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993 Nov 19;75(4):817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]