Abstract
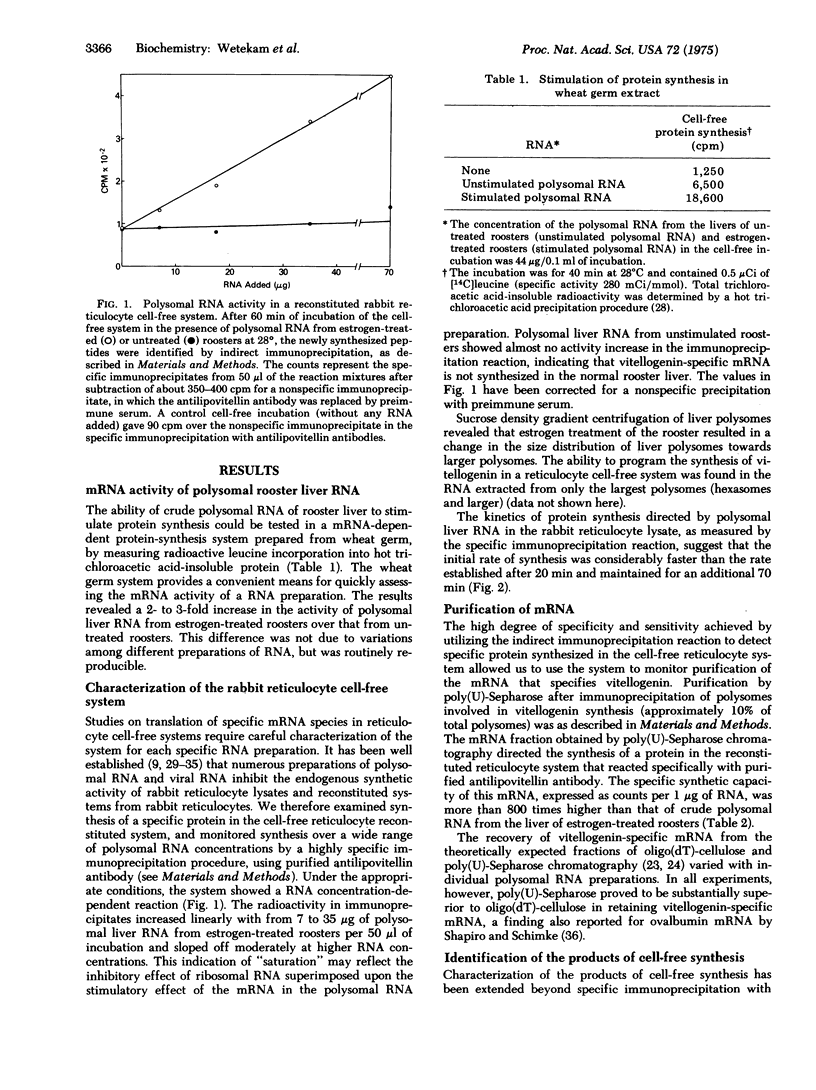
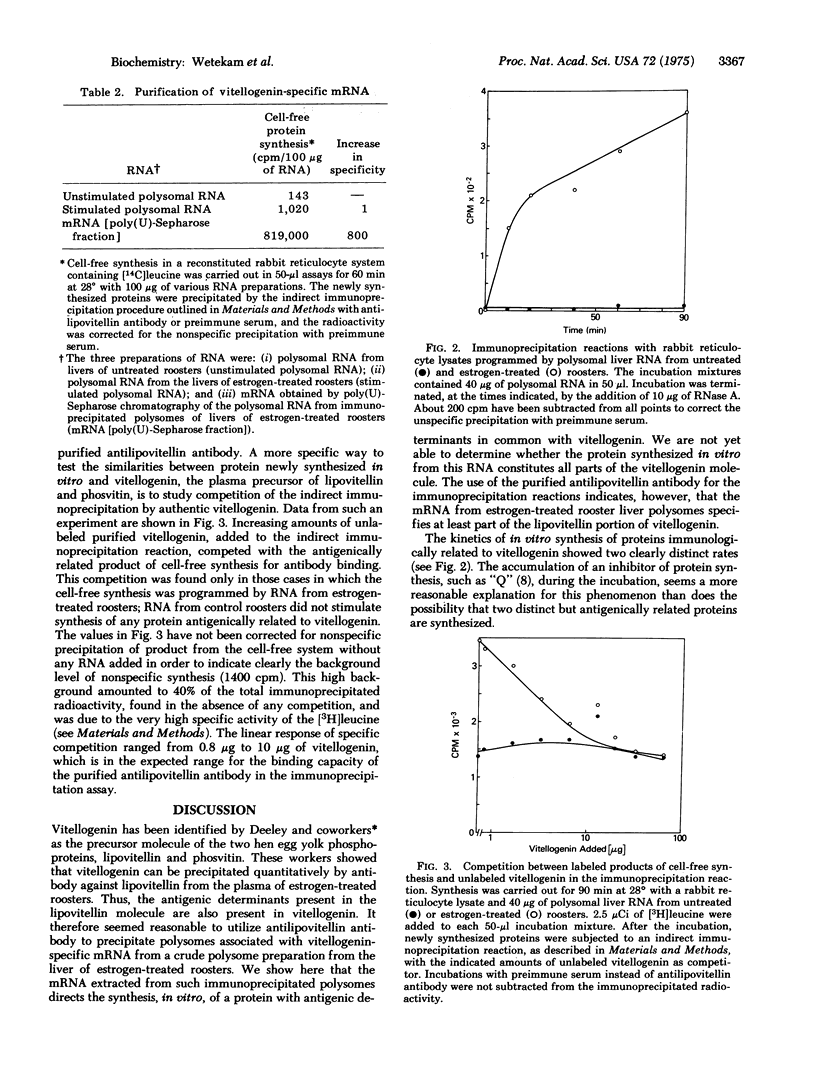
We report initial studies on estrogen-mediated regulation of egg yolk protein synthesis in the rooster. Egg yolk proteins are normally synthesized as a large precursor, vitellogenin, in the liver of the laying hen; roosters synthesize vitellogenin only when treated with estrogen. Polysomal RNA from the liver of estrogen-treated roosters was translated in a reticulocyte cell-free system, and the newly synthesized proteins were identified by a highly specific and sensitive indirect immunoprecipitation reaction. The messenger RNA that specifies vitellogenin has been purified more than 800-fold from rooster liver polysomal RNA by a combination of methods, including immunoprecipitation of polysomes and chromatography of RNA on poly(U)-Sepharose.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axel R., Cedar H., Felsenfeld G. Synthesis of globin ribonucleic acid from duck-reticulocyte chromatin in vitro. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2029–2032. doi: 10.1073/pnas.70.7.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett T., Maryanka D., Hamlyn P. H., Gould H. J. Nonhistone proteins control gene expression in reconstituted chromatin. Proc Natl Acad Sci U S A. 1974 Dec;71(12):5057–5061. doi: 10.1073/pnas.71.12.5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergink E. W., Wallace R. A. Precursor-product relationship between amphibian vitellogenin and the yolk proteins, lipovitellin and phosvitin. J Biol Chem. 1974 May 10;249(9):2897–2903. [PubMed] [Google Scholar]
- Berns A. J., Strous G. J., Bloemendal H. Heterologous in vitro synthesis of lens -crystallin polypeptide. Nat New Biol. 1972 Mar 1;236(61):7–9. doi: 10.1038/newbio236007a0. [DOI] [PubMed] [Google Scholar]
- Falvey A. K., Staehelin T. Structure and function of mammalian ribosomes. I. Isolation and characterization of active liver ribosomal subunits. J Mol Biol. 1970 Oct 14;53(1):1–19. doi: 10.1016/0022-2836(70)90042-2. [DOI] [PubMed] [Google Scholar]
- Gallwitz D., Breindl M. Synthesis of histones in a rabbit reticulocyte cell-free system directed by a polyribosomal RNA fraction from synchronized HeLa cells. Biochem Biophys Res Commun. 1972 Jun 9;47(5):1106–1111. doi: 10.1016/0006-291x(72)90948-5. [DOI] [PubMed] [Google Scholar]
- Gilbert J. M., Anderson W. F. Cell-free hemoglobin synthesis. II. Characteristics of the transfer ribonucleic acid-dependent assay system. J Biol Chem. 1970 May 10;245(9):2342–2349. [PubMed] [Google Scholar]
- Gilmour R. S., Paul J. Tissue-specific transcription of the globin gene in isolated chromatin. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3440–3442. doi: 10.1073/pnas.70.12.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost J. P., Pehling G., Baca O. G. Rate of synthesis of beta L-lipovitellin in the liver of immature chicks treated with 17beta estradiol. Biochem Biophys Res Commun. 1975 Feb 17;62(4):957–965. doi: 10.1016/0006-291x(75)90416-7. [DOI] [PubMed] [Google Scholar]
- Leder P., Honjo T., Packman S., Swan D., Nau M., Norman B. The organization and diversity of immunoglobulin genes. Proc Natl Acad Sci U S A. 1974 Dec;71(12):5109–5115. doi: 10.1073/pnas.71.12.5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg U., Persson T., Philipson L. Isolation and characterization of adenovirus messenger ribonucleic acid in productive infection. J Virol. 1972 Nov;10(5):909–919. doi: 10.1128/jvi.10.5.909-919.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockard R. E., Lingrel J. B. The synthesis of mouse hemoglobin beta-chains in a rabbit reticulocyte cell-free system programmed with mouse reticulocyte 9S RNA. Biochem Biophys Res Commun. 1969 Oct 8;37(2):204–212. doi: 10.1016/0006-291x(69)90720-7. [DOI] [PubMed] [Google Scholar]
- Marcu K., Dudock B. Characterization of a highly efficient protein synthesizing system derived from commercial wheat germ. Nucleic Acids Res. 1974 Nov;1(11):1385–1397. doi: 10.1093/nar/1.11.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews M. B., Korner A. The inhibitory action of a mammalian viral RNA on the initiation of protein synthesis in a reticulocyte cell-free system. Eur J Biochem. 1970 Dec;17(2):339–343. doi: 10.1111/j.1432-1033.1970.tb01171.x. [DOI] [PubMed] [Google Scholar]
- O'Malley B. W., McGuire W. L., Kohler P. O., Korenman S. G. Studies on the mechanism of steroid hormone regulation of synthesis of specific proteins. Recent Prog Horm Res. 1969;25:105–160. doi: 10.1016/b978-0-12-571125-8.50006-5. [DOI] [PubMed] [Google Scholar]
- O'Malley B. W., Means A. R. Female steroid hormones and target cell nuclei. Science. 1974 Feb 15;183(4125):610–620. doi: 10.1126/science.183.4125.610. [DOI] [PubMed] [Google Scholar]
- Oka T., Schimke R. T. Interaction of estrogen and progesterone in chick oviduct development. I. Antagonistic effect of progesterone on estrogen-induced proliferation and differentiation of tubular gland cells. J Cell Biol. 1969 Jun;41(3):816–831. doi: 10.1083/jcb.41.3.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka T., Schimke R. T. Interaction of estrogen and progesterone in chick oviduct development. II. Effects of estrogen and progesterone on tubular gland cell function. J Cell Biol. 1969 Oct;43(1):123–137. doi: 10.1083/jcb.43.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Christensen A. K., Schimke R. T. Organization of polysomes from pre-existing ribosomes in chick oviduct by a secondary administration of either estradiol or progesterone. J Biol Chem. 1970 Feb 25;245(4):833–845. [PubMed] [Google Scholar]
- Palmiter R. D. Magnesium precipitation of ribonucleoprotein complexes. Expedient techniques for the isolation of undergraded polysomes and messenger ribonucleic acid. Biochemistry. 1974 Aug 13;13(17):3606–3615. doi: 10.1021/bi00714a032. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Oka T., Schimke R. T. Modulation of ovalbumin synthesis by estradiol-17 beta and actinomycin D as studied in explants of chick oviduct in culture. J Biol Chem. 1971 Feb 10;246(3):724–737. [PubMed] [Google Scholar]
- Palmiter R. D. Ovalbumin messenger ribonucleic acid translation. Comparable rates of polypeptide initiation and elongation on ovalbumin and globin messenger ribonucleic acid in a rabbit reticulocyte lysate. J Biol Chem. 1973 Mar 25;248(6):2095–2106. [PubMed] [Google Scholar]
- RADOMSKI M. W., COOK W. H. CHROMATOGRAPHIC SEPARATION OF PHOSVITIN, ALPHA- AND BETA-LIPOVITELLIN OF EGG YOLK GRANULES ON TEAE-CELLULOSE. Can J Biochem. 1964 Aug;42:1203–1215. doi: 10.1139/o64-130. [DOI] [PubMed] [Google Scholar]
- Rhoads R. E., McKnight G. S., Schimke R. T. Quantitative measurement of ovalbumin messenger ribonucleic acid activity. Localization in polysomes, induction by estrogen, and effect of actinomycin D. J Biol Chem. 1973 Mar 25;248(6):2031–2039. [PubMed] [Google Scholar]
- Rhoads R. E., McKnight G. S., Schimke R. T. Synthesis of ovalbumin in a rabbit reticulocyte cell-free system programmed with hen oviduct ribonucleic acid. J Biol Chem. 1971 Dec 10;246(23):7407–7410. [PubMed] [Google Scholar]
- Rosenfeld G. C., Comstock J. P., Means A. R., O'Malley B. W. Estrogen-induced synthesis of ovalbumin messenger RNA and its translation in a cell-free system. Biochem Biophys Res Commun. 1972 Feb 25;46(4):1695–1703. doi: 10.1016/0006-291x(72)90805-4. [DOI] [PubMed] [Google Scholar]
- Schreier M. H., Staehelin T. Initiation of mammalian protein synthesis: the importance of ribosome and initiation factor quality for the efficiency of in vitro systems. J Mol Biol. 1973 Feb 19;73(3):329–349. doi: 10.1016/0022-2836(73)90346-x. [DOI] [PubMed] [Google Scholar]
- Shapiro D. J., Schimke R. T. Immunochemical isolation and characterization of ovalbumin messenger ribonucleic acid. J Biol Chem. 1975 Mar 10;250(5):1759–1764. [PubMed] [Google Scholar]
- Shapiro D. J., Taylor J. M., McKnight G. S., Palacios R., Gonzalez C., Kiely M. L., Schimke R. T. Isolation of hen oviduct ovalbumin and rat live albumin polysomes by indirect immunoprecipitation. J Biol Chem. 1974 Jun 25;249(12):3665–3671. [PubMed] [Google Scholar]
- Sheiness D., Darnell J. E. Polyadenylic acid segment in mRNA becomes shorter with age. Nat New Biol. 1973 Feb 28;241(113):265–268. doi: 10.1038/newbio241265a0. [DOI] [PubMed] [Google Scholar]
- Sippel A. E., Stavrianopoulos J. G., Schutz G., Feigelson P. Translational properties of rabbit globin mRNA after specific removal of poly(A) with ribonuclease H. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4635–4639. doi: 10.1073/pnas.71.11.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soreq H., Nudel U., Salomon R., Revel M., Littauer U. Z. In vitro translation of polyadenylic acid-free rabbit globin messenger RNA. J Mol Biol. 1974 Sep 5;88(1):233–245. doi: 10.1016/0022-2836(74)90307-6. [DOI] [PubMed] [Google Scholar]
- Stavnezer J., Huang R. C. Synthesis of a mouse immunoglobulin light chain in a rabbit reticulocyte cell-free system. Nat New Biol. 1971 Apr 7;230(14):172–176. doi: 10.1038/newbio230172a0. [DOI] [PubMed] [Google Scholar]
- Wallace R. A., Jared D. W. Studies on amphibian yolk. 8. The estrogen-induced hepatic synthesis of a serum lipophosphoprotein and its selective uptake by the ovary and trasformation into yolk platelet proteins in Xenopus laevis. Dev Biol. 1969 May;19(5):498–526. doi: 10.1016/0012-1606(69)90085-2. [DOI] [PubMed] [Google Scholar]
- Wallace R. A. Studies on amphibian yolk. IX. Xenopus vitellogenin. Biochim Biophys Acta. 1970 Jul 21;215(1):176–183. doi: 10.1016/0304-4165(70)90400-9. [DOI] [PubMed] [Google Scholar]
- Williamson R. Properties of rapidly labelled deoxyribonucleic acid fragments isolated from the cytoplasm of primary cultures of embryonic mouse liver cells. J Mol Biol. 1970 Jul 14;51(1):157–168. doi: 10.1016/0022-2836(70)90277-9. [DOI] [PubMed] [Google Scholar]


