Abstract
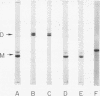
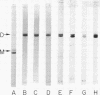
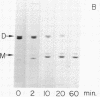
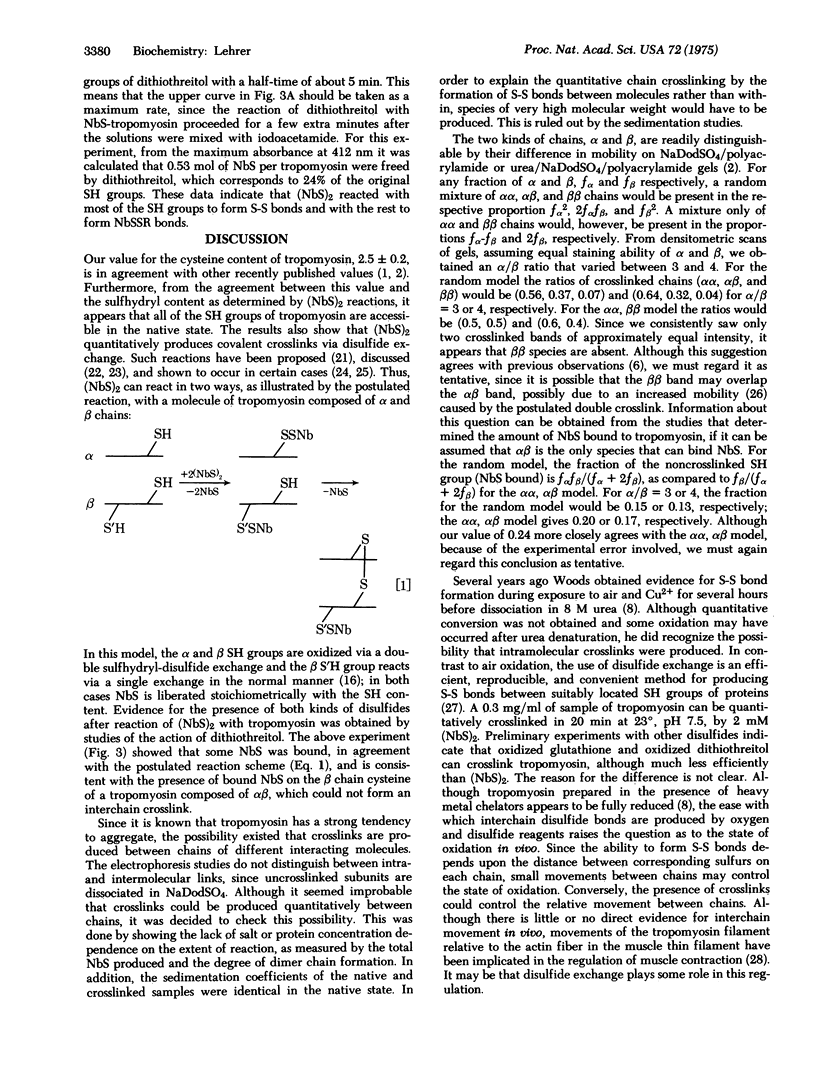
Rabbit skeletal muscle tropomyosin can be crosslinked in the native state by the use of 5,5'-dithiobis(2-nitrobenzoate), which forms disulfide bonds between the two subunits. Using polyacrylamide gel electrophoresis in sodium dodecyl sulfate we have shown that this crosslinking goes to completion over a wide range of protein concentration, ionic strength, and reagent concentration. Crosslinks are not formed in denaturing solvents such as sodium dodecyl sulfate and guanidine hydrochloride despite the fact that the same number of SH groups react as in the native state (2.3 +/- 0.2). The sedimentation coefficients of crosslinked and non-crosslinked samples are identical. Thus, crosslinks are formed between corresponding cysteines on different chains of the same molecule. This provides strong evidence for a model of chain interaction with both chains in register. Evidence has also been obtained that rabbit skeletal tropomyosin is composed only of alphaalpha and alphabeta subunits rather than a random mixture of chains.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey K. Tropomyosin: a new asymmetric protein component of the muscle fibril. Biochem J. 1948;43(2):271–279. doi: 10.1042/bj0430271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Little G. Reactions of papain and of low-molecular-weight thiols with some aromatic disulphides. 2,2'-Dipyridyl disulphide as a convenient active-site titrant for papain even in the presence of other thiols. Biochem J. 1973 May;133(1):67–80. doi: 10.1042/bj1330067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connellan J. M., Folk J. E. Mechanism of the inactivation of guinea pig liver transglutaminase by 5,5'-dithiobis-(2-nitrobenzoic acid). J Biol Chem. 1969 Jun 25;244(12):3173–3181. [PubMed] [Google Scholar]
- Cummins P., Perry S. V. The subunits and biological activity of polymorphic forms of tropomyosin. Biochem J. 1973 Aug;133(4):765–777. doi: 10.1042/bj1330765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- FERNANDEZDIEZ M. J., OSUGA D. T., FEENEY R. E. THE SULFHYDRYLS OF AVIAN OVALBUMINS, BOVINE BETA-LACTOGLOBULIN, AND BOVINE SERUM ALBUMIN. Arch Biochem Biophys. 1964 Sep;107:449–458. doi: 10.1016/0003-9861(64)90301-7. [DOI] [PubMed] [Google Scholar]
- Glazer A. N. Specific chemical modification of proteins. Annu Rev Biochem. 1970;39:101–130. doi: 10.1146/annurev.bi.39.070170.000533. [DOI] [PubMed] [Google Scholar]
- Greaser M. L., Gergely J. Reconstitution of troponin activity from three protein components. J Biol Chem. 1971 Jul 10;246(13):4226–4233. [PubMed] [Google Scholar]
- Griffith I. P. The effect of cross-links on the mobility of proteins in dodecyl sulphate-polyacrylamide gels. Biochem J. 1972 Feb;126(3):553–560. doi: 10.1042/bj1260553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges R. S., Smillie L. B. Cyanogen bromide fragments of rabbit skeletal tropomyosin. Can J Biochem. 1973 Jan;51(1):56–70. doi: 10.1139/o73-008. [DOI] [PubMed] [Google Scholar]
- Hodges R. S., Smillie L. B. Cysteine sequences of rabbit skeletal tropomyosin. Can J Biochem. 1972 Mar;50(3):330–343. doi: 10.1139/o72-045. [DOI] [PubMed] [Google Scholar]
- Hodges R. S., Smillie L. B. The histidine and methionine sequences of rabbit skeletal tropomyosin. Can J Biochem. 1972 Mar;50(3):312–329. doi: 10.1139/o72-044. [DOI] [PubMed] [Google Scholar]
- Johnson F., Smillie L. B. Rabbit skeletal alpha-tropomyosin chains are in register. Biochem Biophys Res Commun. 1975 Jun 16;64(4):1316–1322. doi: 10.1016/0006-291x(75)90836-0. [DOI] [PubMed] [Google Scholar]
- KOMINZ D. R., SAAD F., GLADNER J. A., LAKI K. Mammalian tropomyosins. Arch Biochem Biophys. 1957 Jul;70(1):16–28. doi: 10.1016/0003-9861(57)90075-9. [DOI] [PubMed] [Google Scholar]
- Kleppe K., Damjanovich S. Studies on the SH groups of phosphorylase b. Reaction with 5,5'-dithiobis-(2-nitrobenzoic acid). Biochim Biophys Acta. 1969 Jul 8;185(1):88–102. doi: 10.1016/0005-2744(69)90285-x. [DOI] [PubMed] [Google Scholar]
- Pont M. J., Woods E. F. Denaturation of tropomyosin by guanidine hydrochloride. Int J Protein Res. 1971;3(4):177–183. doi: 10.1111/j.1399-3011.1971.tb01710.x. [DOI] [PubMed] [Google Scholar]
- Sodek J., Hodges R. S., Smillie L. B., Jurasek L. Amino-acid sequence of rabbit skeletal tropomyosin and its coiled-coil structure. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3800–3804. doi: 10.1073/pnas.69.12.3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wetlaufer D. B., Ristow S. Acquisition of three-dimensional structure of proteins. Annu Rev Biochem. 1973;42:135–158. doi: 10.1146/annurev.bi.42.070173.001031. [DOI] [PubMed] [Google Scholar]
- Woods E. F. Comparative physicochemical studies on vertebrate tropomyosins. Biochemistry. 1969 Nov;8(11):4336–4344. doi: 10.1021/bi00839a017. [DOI] [PubMed] [Google Scholar]
- Woods E. F. Molecular weight and subunit structure of tropomyosin B. J Biol Chem. 1967 Jun 25;242(12):2859–2871. [PubMed] [Google Scholar]
- Yamaguchi M., Greaser M. L., Cassens R. G. Interactions of troponin subunits with different forms of tropomyosin. J Ultrastruct Res. 1974 Jul;48(1):33–58. doi: 10.1016/s0022-5320(74)80043-2. [DOI] [PubMed] [Google Scholar]