Abstract
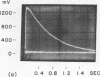
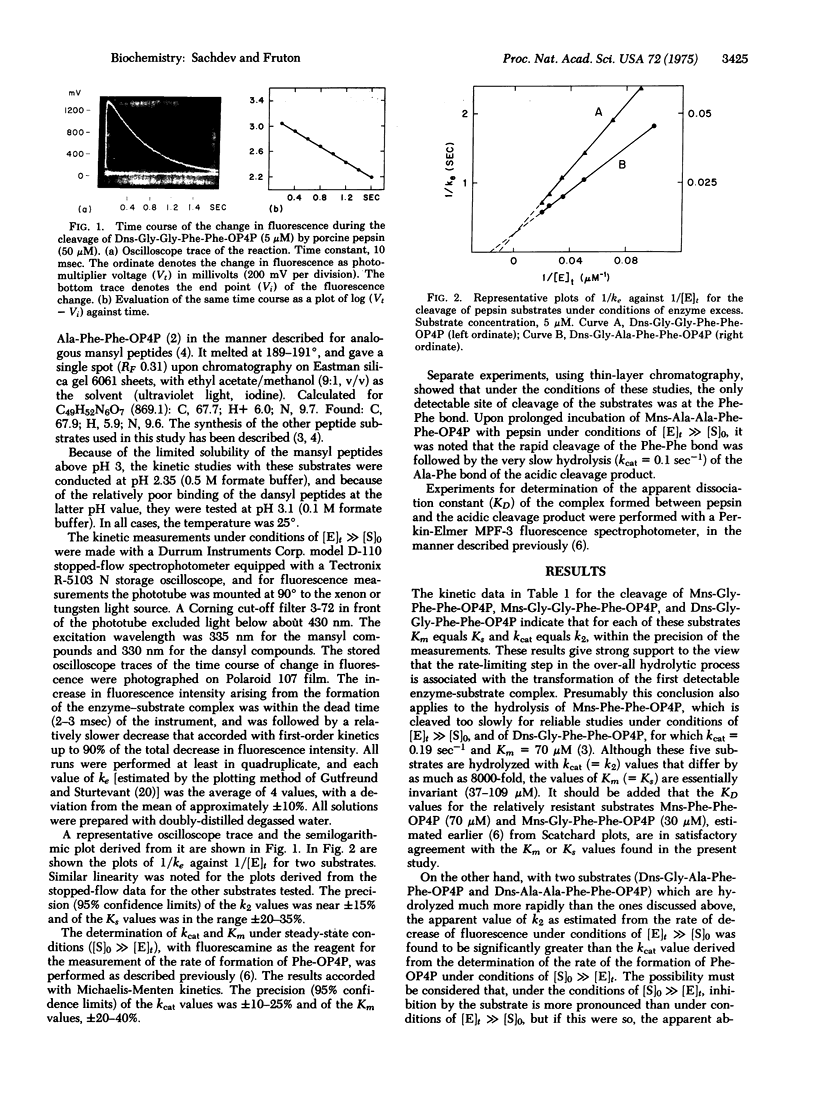
Oligopeptide substrates of porcine pepsin (E) of the type A-Phe-Phe-B (S) that are cleaved solely at the Phe-Phe bond under the conditions of these studies, and bearing an amino-terminal fluorescent probe group (mansyl or dansyl), have been used for stopped-flow measurements of the rate of formation of the A-Phe product. These experiments were conducted under conditions of [E] greater than [S], and the kinetic data were compared with those obtained under conditions of [S] greater than [E] for the formation of the Phe-B product (the same in all cases). The results for substrates with A = mansyl-Gly, mansyl-Gly-Gly, and dansyl-Gly-Gly support the conclusion that the rate-limiting step in the over-all catalytic process is associated with the scission of the Phe-Phe bond in the first detectables ES complex. Although the rate of this step varies widely with the nature of the A portion of A-Phe-Phe-B, the magnitude of the dissociation constant of ES is relatively invariant. This supports the view that, in the cleavage of oligopeptide substrates by pepsin, secondary enzyme--substrate interactions may cause conformational changes at the catalytic site, and that a portion of the total binding energy may be used for the attainment of the transition state in the bond-breaking step. With substrates that are hydrolyzed extremely rapidly (A = dansyl-Gly-Ala, dansyl-Ala-Ala), the rate of formation of the A-Phe product appears to be faster than the steady-state rate, suggesting that an additional step has become kinetically significant in the over-all process. This step may be associated with the return of the conformation of the active site to its original state.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Delpierre G. R., Fruton J. S. Inactivation of pepsin by diphenyldiazomethane. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1161–1167. doi: 10.1073/pnas.54.4.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fersht A. R. Catalysis, binding and enzyme-substrate complementarity. Proc R Soc Lond B Biol Sci. 1974 Nov 19;187(1089):397–407. doi: 10.1098/rspb.1974.0084. [DOI] [PubMed] [Google Scholar]
- GUTFREUND H., STURTEVANT J. M. The mechanism of the reaction of chymotrypsin with p-nitrophenyl acetate. Biochem J. 1956 Aug;63(4):656–661. doi: 10.1042/bj0630656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham J. E., Sodek J., Hofmann T. Rhizopus acid proteinases (rhizopus-pepsins): properties and homology with other acid proteinases. Can J Biochem. 1973 Jun;51(6):789–796. doi: 10.1139/o73-098. [DOI] [PubMed] [Google Scholar]
- Hiroara H., Bender M. L., Stark R. S. Acylation of alpha-chymotrypsin by oxygen and sulfur esters of specific substrates: kinetic evidence for a tetrahedral intermediate. Proc Natl Acad Sci U S A. 1974 May;71(5):1643–1647. doi: 10.1073/pnas.71.5.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard C. D., Kirsch J. F. The presteady-state kinetics of the papain-catalyzed hydrolysis of isomeric nitrophenyl esters of carbobenzoxyglycine. Biochemistry. 1968 Jul;7(7):2569–2573. doi: 10.1021/bi00847a018. [DOI] [PubMed] [Google Scholar]
- Inouye K., Fruton J. S. The inhibition of pepsin action. Biochemistry. 1968 May;7(5):1611–1615. doi: 10.1021/bi00845a001. [DOI] [PubMed] [Google Scholar]
- KEZDY F. J., BENDER M. L. The kinetics of the alpha-chymotrypsin-catalyzed hydrolysis of p-nitrophenyl acetate. Biochemistry. 1962 Nov;1:1097–1106. doi: 10.1021/bi00912a021. [DOI] [PubMed] [Google Scholar]
- Page M. I., Jencks W. P. Entropic contributions to rate accelerations in enzymic and intramolecular reactions and the chelate effect. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1678–1683. doi: 10.1073/pnas.68.8.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan T. G., Moore S., Stein W. H. Pepsin from pepsinogen. Preparation and properties. J Biol Chem. 1966 Nov 10;241(21):4940–4950. [PubMed] [Google Scholar]
- Raju E. V., Humphreys R. E., Fruton J. S. Gel filtration studies on the binding of peptides to pepsin. Biochemistry. 1972 Sep 12;11(19):3533–3536. doi: 10.1021/bi00769a007. [DOI] [PubMed] [Google Scholar]
- Sachdev G. P., Brownstein A. D., Fruton J. S. Fluorescence studies on the active sites of porcine pepsin and Rhizopus-pepsin. J Biol Chem. 1975 Jan 25;250(2):501–507. [PubMed] [Google Scholar]
- Sachdev G. P., Brownstein A. D., Fruton J. S. N-methyl-2-anilinonaphthalene-6-sulfonyl peptides as fluorescent probes for pepsin-substrate interaction. J Biol Chem. 1973 Sep 25;248(18):6292–6299. [PubMed] [Google Scholar]
- Sachdev G. P., Fruton J. S. Pyridyl esters of peptides as synthetic substrates of pepsin. Biochemistry. 1969 Nov;8(11):4231–4238. doi: 10.1021/bi00839a002. [DOI] [PubMed] [Google Scholar]
- Sachdev G. P., Fruton J. S. Secondary enzyme-substrate interactions and the specificity of pepsin. Biochemistry. 1970 Nov 10;9(23):4465–4470. doi: 10.1021/bi00825a001. [DOI] [PubMed] [Google Scholar]
- Sachdev G. P., Johnston M. A., Fruton J. S. Fluorescence studies on the interaction of pepsin with its substrates. Biochemistry. 1972 Mar 14;11(6):1080–1086. doi: 10.1021/bi00756a021. [DOI] [PubMed] [Google Scholar]
- Sampath-Kumar P. S., Fruton J. S. Studies on the extended active sites of acid proteinases. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1070–1072. doi: 10.1073/pnas.71.4.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver M. S., Stoddard M. Amino-enzyme intermediates in pepsin-catalyzed reactions. Biochemistry. 1972 Jan 18;11(2):191–200. doi: 10.1021/bi00752a008. [DOI] [PubMed] [Google Scholar]
- Silver M. S., Stoddard M. Kinetic studies on the mechanism of pepsin action. Biochemistry. 1975 Feb 11;14(3):614–621. doi: 10.1021/bi00674a023. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Wang T. T., Hofmann T. Acyl intermediates in pepsin and penicillopepsin catalyzed reactions. Biochem Biophys Res Commun. 1974 Mar 15;57(1):39–46. doi: 10.1016/s0006-291x(74)80354-2. [DOI] [PubMed] [Google Scholar]