Abstract
Ketamine is widely used as an anesthetic, analgesic, or sedative in pediatric patients. We reported that ketamine alters the normal neurogenesis of rat fetal neural stem progenitor cells (NSPCs) in the developing brain, but the underlying mechanisms remain unknown. The PI3K-PKB/Akt (Phosphatidylinositide 3-kinases/protein kinase B) signaling pathway plays many important roles in cell survival, apoptosis, and proliferation. We hypothesized that PI3K-PKB/Akt signaling may be involved in ketamine-altered neurogenesis of cultured NSPCs in vitro. NSPCs were isolated from Sprague-Dawley rat fetuses on gestational day 17. BrdU (bromodeoxyuridine) incorporation, Ki67 staining, and differentiation tests were utilized to identify primary cultured NSPCs. Immunofluorescent staining was used to detect Akt expression, whereas, Western blots measured phosphorylated Akt and p27 expression in NSPCs exposed to different treatments. We report that cultured NSPCs had properties of neurogenesis: proliferation and neural differentiation. PKB/Akt was expressed in cultured rat fetal cortical NSPCs. Ketamine inhibited the phosphorylation of Akt and further enhanced p27 expression in cultured NSPCs. All ketamine-induced PI3K/Akt signaling changes could be recovered by NMDA (N-Methyl-D-aspartate) receptor agonist, NMDA. These data suggest that inhibition of PI3K/Akt-p27 signaling may be involved in ketamine-induced neurotoxicity in the developing brain, whereas excitatory NMDA receptor activation may reverse these effects.
Introduction
Ketamine is widely used as an anesthetic, analgesic, and sedative in pediatric clinical settings and is consumed as an abuse drug in many countries. Thus, the developing brains of pediatric patients and fetuses of pregnant drug abusers could be exposed to high doses or prolonged periods of ketamine. A series of experiments have unraveled the effects of ketamine on cell death and neurogenesis of rat fetal cortical neurons and neural stem progenitor cells (NSPCs)(Dong et al., 2012; Ikonomidou et al., 1999; Wang et al., 2005; Wang et al., 2006; Zou et al., 2009). Our studies found that NSPCs were resistant to the ketamine-induced neurotoxicity and ketamine inhibited the proliferation and promoted neuronal differentiation in NPSCs in concentration- and time-dependent manners (Dong et al., 2012). However, the underlying mechanism of these effects remains unknown.
The phosphatidylinositol 3-kinase (PI3K) signaling pathway serves a central role in a variety of important physiological processes, associated with cell survival(Cross et al., 1995; Crowder and Freeman, 1998; Dudek et al., 1997; Pap and Cooper, 1998), proliferation(Bruel-Jungerman et al., 2009), protein synthesis, cell growth, glycogen metabolism, morphology, migration and angiogenesis(Kennedy et al., 1997; Manning and Cantley, 2007). PKB/Akt is highly expressed in the mammalian central nervous system, which is consistent with its importance in neuronal function(Sutton and Chandler, 2002). The PI3K/Akt signaling pathway participates in some important cellular activities mediated by NMDA (N-Methyl-D-aspartate) receptors and also modulates the activity of NMDA receptors directly. Inhibition of the PI3K/Akt signaling pathway was implicated in the developmental neurotoxicity NMDA receptor antagonists like PCP(Lei et al., 2008; Xia et al., 2010; Xia et al., 2008). The PI3K/Akt signaling system also serves a protective role in cell survival and the proliferation, acting in concert with other signaling pathways related to the regulation of apoptosis, cell cycle, and differentiation. The neuroprotective effects of growth factors and mitogens such as nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), transforming growth factor (TGF)-beta1, and even insulin are mediated by the activation of PI3K/Akt pathway in NSPCs(Nguyen et al., 2009; Park et al., 2008; Zhao et al., 2007). Thus, it is likely that PI3K/Akt signaling is also involved in the ketamine-induced changes in neurogenesis from NSPCs.
PI3K/Akt stimulates the proliferation of NSPCs through multiple downstream targets on cell cycle regulation(Gan et al., 2008; Paling et al., 2004; Sinor and Lillien, 2004; Watanabe et al., 2006). Akt phosphorylates p27Kip1, a G1 cyclin-dependent kinase inhibitor(Brennan et al., 2002; Dijkers et al., 2000; Murillo et al., 2001; Sherr and Roberts, 1999; Sun et al., 1999), which results in its cytosolic sequestration via 14-3-3 binding(Sekimoto et al., 2004). This prevents p27 localization to the nucleus and attenuates its cell cycle inhibitory effects. Mutation of the phosphorylation of p27 can block the proliferative effects of various constitutively active Akt derivatives(Liang et al., 2002; Shin et al., 2002; Viglietto et al., 2002). Conversely, activation of Akt inhibits p27 and cyclin D1 through phosphorylation and inhibition of the FOXO transcription factors in the extracellular growth factors environment(Medema et al., 2000). We hypothesized, therefore, ketamine-induced changes in proliferation and differentiation of NSPCs may be mediated via the PI3K/Akt/p27 signaling pathway. To test this hypothesis, our experiments were designed to detect changes in the phosphorylation of Akt proteins and its downstream factor p27 in the NSPCs exposed to ketamine.
Materials and Methods
Animal
Timed-pregnant Sprague-Dawley (SD) rats were commercially purchased from Charles River Inc. Rats were housed at 24°C on a 12:12 hours light:dark cycle with free access to food and water. All animal use procedures were approved by the IACUC (Institutional Animal Care and Use Committee) of the University of Tennessee Health Science Center and complied with the ethical standards described in the NIH Guide for Laboratory Animals.
Cell culture
Isolation procedures for NSPCs were completed as reported previously(Dong et al., 2012). Briefly, 9–12 rat fetuses at embryonic day 17 were obtained from one time pregnant rat at gestational day 17. All neocortices from E17 rats were dissected, collected and cut into small pieces for digestion in Accutase™ (A6964, Sigma-Aldrich) at 37ºC; dissociated cells were rinsed with Dulbecco’s Phosphate-Buffered Saline (DPBS, 14190, Invitrogen) and centrifuged at 120 × g for 5min. Cell pellets were gently re-suspended in the proliferation medium DMEM/F12 (11330, Invitrogen) containing 20 ng/ml each of epidermal growth factor (EGF, 236-EG, R&D) and basic fibroblast growth factor (bFGF, 233-FB, R&D). Sieved cell suspensions were mixed with Percoll™ ( P4937, Sigma-Aldrich) centrifugation medium, centrifuged at 21000 × g for 30min for separation into two cellular layers. Cells from the lower layer were rinsed and suspended in proliferation medium. Isolated cells were seeded on poly-L-lysine (PLL, P4707, Sigma-Aldrich) pre-coated 60 mm cell culture dishes (12-565-97, Thermo Scientific) or 6-well tissue culture plates (07-200-83, Corning) at a density of 1×106 cells /well (dish). Seeded cells were cultured in 5% CO2, 100% humidity at 37ºC. In each dish, half of the culture medium was replaced every three days. Adherent cells were passaged using Accutase™. Cultures at passage 1~2 were used for all in vitro experiments.
NSPCs identification assays
To identify the proliferation potency of NSPCs, BrdU (5-bromo-2'-deoxyuridine) incorporation assays and Ki67 were used as proliferation specific biomarker. NSPCs cultures were exposed to 10 μM of BrdU (AC228590025, ACROS) for 24 hours. Treated cells were fixed in 4% PFA (polyformaldehyde, 30525-89-4, ACROS) for immunofluorescent staining using anti-BrdU (MAB4072, Millipore) and anti-Ki67 (AB9260, Millipore) antibodies to detect proliferating and proliferative cells. To investigate their differentiation capacity, NSPCs were cultured in differentiation medium (containing 1% fetal bovine serum (FBS, SH30070.03, Hyclone) and without mitogens-bFGF and EGF) for 2 weeks. Then, the cultures were fixed in 4% PFA and then stained using anti-GFAP (AB5804, Millipore) and anti-Tuj-1 (MAB5564, Millipore) to label astrocytes and neurons, respectively. All nuclei were stained with 1 μg/ml of 4’, 6’-diamidion-2’-phenylindole (DAPI, 46190, Thermo Scientific)
Immunofluorescent staining
Immunofluorescent staining methods were used to detect the expression of Akt, Ki67, Tuj-1, and GFAP and BrdU incorporation in this study using anti-Akt (05-591, Millipore), anti-Ki67 (AB9260, Millipore), anti-Tuj-1 (MAB5564, Millipore), anti-GFAP (AB5804, Millipore), and anti-BrdU (MAB4072, Millipore) antibodies. Specifically, cultures or sliced neurosphere sections (10 μm) were fixed in 4% PFA for 30 min, placed in blocking buffer (5% goat serum) for 40 min and incubated in diluted primary antibodies (dilution prepared in PBS containing 5% BSA (BP1600-100, Fisher BioReagents)) overnight at 4 ºC. Cultures were rinsed in PBST (PBS containing 1% Triton X-100 (BP151, Fisher BioReagents)) three times for 10 min for each. Then, samples were incubated in secondary antibodies (Rabbit IgG Alexa 594/ Mouse IgG Alexa 488, Invitrogen) for 1 hour at room temperature followed by DAPI incubation (1 μg/ml) for 10 min at room temperature. Finally, cultures were rinsed with PBST three times for 10 min for each, and mounted in aqueous mounting medium (ab128982, Abcam). Images of the slides were captured using an Olympus BX60 upright fluorescent microscope (Olympus Inc., Japan) with Hamamatsu imaging system (Hamamatsu C4742-95 camera and Imaging program-HCImage 2.1 Live Version, Hamamatsu Photonics Inc., Japan).
Cell treatments
To determine the concentration responses of ketamine on the expression of phosphorylated Akt and p27, NSPCs cultures (1×106 cells /well) in a 6-well plate were treated with ketamine (Ketaset® Fort Dodge, USK) at different concentrations (0, 1, 10, 20, 50, and 100 μM) for 24 h. Protein samples of treated NSPCs cultures were prepared using cell lysis buffer and collected for Western blot tests. The expression of phosphorylated Akt and p27 following exposure to different concentrations of ketamine was detected using standard Western blot protocols with anti-Akt (05-591, Millipore), anti-phosphorylated Akt (05-669, Millipore), and anti-p27 (06-445, Millipore) antibodies.
To determine whether the expression of p27 can be regulated by phosphorylation of Akt protein in NSPCs, the PI3K/Akt signaling pathway inhibitor, LY-294002 (L9908, Sigma-Aldrich), was used to block the activation of Akt. Prepared NSPCs (1×106 cells /well) were exposed to vehicle and 10 μM concentrations of LY-294002 for 24 hrs. The changes in phosphorylated Akt and p27 were detected using Western blot methods.
To identify whether ketamine-induced changes in the expression of p-Akt in cultured NSPCs were mediated by NMDA receptors, NMDA, the receptor ligand, was used to block the ketamine-induced decrease of Akt phosphorylation. Four experimental groups were set up using Vehicle, NMDA (50 μM, 6384-92-5, ACROS Organics), ketamine (10 μM), and NMDA (50 μM) plus ketamine (10 μM). Based on previous publication(Sinner and Graf, 2008), a ketamine blood level for a general anesthesia is 2000–3000 ng/ml, which is around 10 μM. Therefore, we choose 10 μM of ketamine as a clinically relevant concentration in these assays. After 24 hours exposure, cultures (1×106 cells /well) were lysed with cell lysis buffer and proteins were extracted for western-blot tests.
Western blot methods
Proteins were extracted from treated cells using RIPA cell lysis buffer (20-188, Millipore) containing Halt Protease Inhibitor Cocktail (1861280, Thermo Scientific). Pierce MicroBCA kit (PI23235, Peirce) was employed to determine total protein concentrations; 20 μg of proteins from each sample were loaded for running SDS-PAGE. Then, separated proteins were transferred onto a PVDF membrane (509013239, Millipore). After blocking in 5% BSA in TBST (Tris-buffered saline containing 1% Tween-20 (170-6531, Bio-Rad)), membranes were incubated with primary antibody diluted in TBST containing 5% BSA on a rocker plate overnight at 4 ºC. Membranes were washed 3 times for a minimum of 5 minutes each in TBST. Then, membranes were incubated in secondary antibody provided in Immn-Star WesternC™ Chemiluminescent Kits (170-5070, Bio-Rad) (1:10000 for chemiluminescence; diluted in TBST containing 5% BSA) for 60 min at room temperature on a rocker plate. After washing with TBST 3 times, HRP substrate and buffer (170-5070, Bio-Rad) were mixed in a ratio of 1:1 and dropped on membranes. After a 1-minute reaction, protein bands were recorded on X-ray films developed using a film developing machine (SRX-101A, Konica).
Data collection and statistical analyses
Each experiment was completed biologically and independently at least three times. Western blots data were analyzed based on multiple independent experiments. Briefly, X-ray films of western blot tests were scanned with a photo scanner and the scanning images were saved as TIFF files. The integrated gray densities of protein bands on the images were measured using ImageJ program (downloaded from the NIH website: http://rsbweb.nih.gov/ij/). The specific protein band was normalized to the corresponding beta-actin band after subtracting the background. Typical image reflecting protein expression changes for each assay were displayed and normalized density measurements were analyzed for group comparisons. All data from multiple independent experiments were analyzed by one-way ANOVA followed by Bonferroni correction for multiple comparisons or a t-test assuming unequal variances. Normalized means and standard deviations are shown in the graphs. Statistical significance was assumed if a null hypothesis could be rejected at P<0.05. All statistical analysis was performed using the following software: Microsoft Excel 2010 (Microsoft Co.) and GraphPad InStat3 (GraphPad Software, Inc.).
Results
NSPCs culture and identification
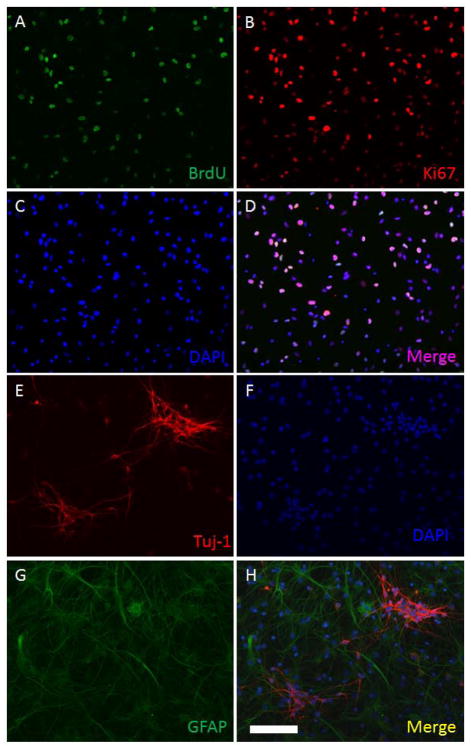
Isolated cells from the rat fetal neocortex were cultured in vitro in DMEM/F12 proliferation medium for 5 days. Adherent cultures were obtained as a monolayer on poly-L-lysine precoated cell culture plates (Figure 1A), whereas free floating neurospheres were formed in non-coated cell culture (Figure 1B). In the BrdU cell proliferation assays, obtained cultures incorporated BrdU after a 24-hour exposure (Figure 2A-D), showing actively proliferating NSPCs in the S-phase of the cell cycle. Ki67 positive staining was found in all cells, indicating all NSPCs have proliferative capacity. (Figure 2A–D) Differentiation assays also showed that all differentiated NSPCs either expressed GFAP or Tuj-1, suggesting the obtained cultures had the potencies to differentiate into astrocytes and neurons. (Figure 2E-H)
Figure 1. Isolation and culture of NSPCs as adherent monolayers and neurospheres.

Isolated cells from rat fetal cortices were in vitro cultured in NSPC proliferation medium for 5 days. Figure 1A, seeded on a poly-L-lysine pre-coated cell culture dish; cultured cells grew as an adherent monolayer. Figure 1B, neurospheres were formed in a non-coating cell culture dish. (Scale bar: 100 μm)
Figure 2. Identification of neurogenesis potencies of NSPC cultures.
Neurogenesis properties (proliferation and differentiation) of NSPCs were identified using BrdU incorporation assays (Images AD) and default differentiation tests (Image E–H). Figure 2A, cultured cells were exposed to 10 μM of BrdU for 24 hrs. BrdU can be incorporated into proliferating cells (green). Figure 2B, all cultured cells were Ki67 positive (red), indicating that the cells were proliferative. Figure 2C, all cellular nuclei were stained by DAPI (blue). Figure 2D, a merge image of Figure 2A–C. Figure 2E, some differentiated cells are stained by neuronal biomarker-Tuj-1 (red). Figure 2F, nuclei staining-DAPI (blue). Image G, some cultured cells differentiated into astrocytes showing GFAP positive (green). Image H, a merge image of Image E–G. (Scale bar: 100 μm)
Expression of Akt in cultured NSPCs
To confirm the existence of PI3K/Akt signaling in NSPCs, neurosphere sections (10 μm) and adherently cultured NSPCs were stained using anti-PKB/Akt antibody. Results showed that the expression of PKB/Akt was detected in neurospheres (Figure 3A–C) and adherently cultured NSPCs (Figure 3D). The expression of Akt in NSPCs suggests that the PI3K/Akt signaling pathway may play a potential role in anti-apoptosis, pro-survival, and regulating cell cycles in the cultured NSPCs.
Figure 3. Expression of Akt in in vitro cultured NSPCs.
NSPCs from neurospheres and adherent monolayer cultures are detected expression of Akt proteins. Figure 3A–C, A, a sectioned neurosphere (10 μm thick) was stained with anti-Akt antibody (red staining); B, DAPI staining (blue); C, a merged image of Figure 3A and 3B. Figure 3D, NSPCs cultured as adherent monolayer were stained with anti-Akt antibody, showing all cells are Akt positive (green). (Scale bar: 50 μm)
Ketamine inhibits PI3K-Akt signaling in a concentration dependent manner
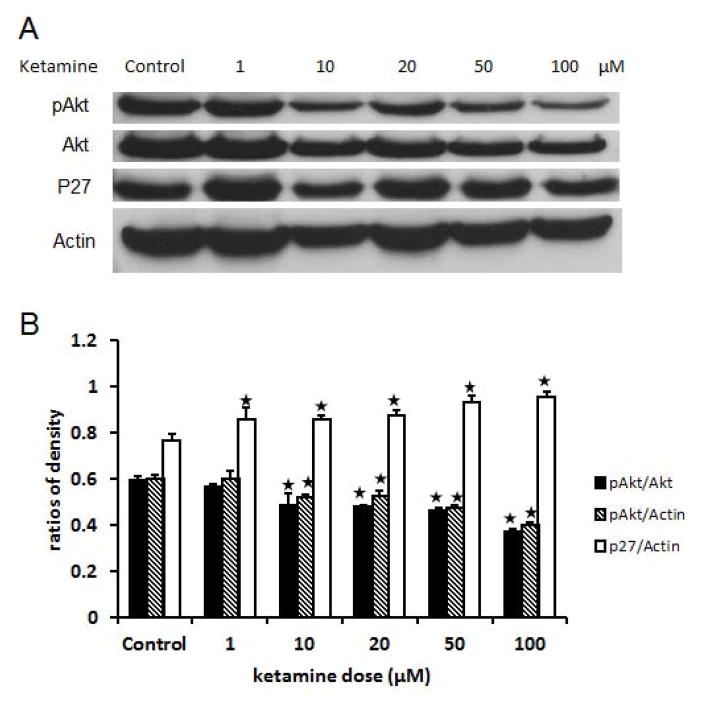
PI3K/Akt signaling pathway plays a critical role in the regulation of neurogenesis in NSPCs. To analyze the role of PI3K/Akt signaling pathway in the regulation of proliferation, this study focused on a key downstream factor of Akt proteins, p27. Based on previous studies, p27 is a cdk kinase inhibitor that negatively regulates the cell cycle, especially at G1/S checkpoint. Here, our results show that ketamine exposure concentration-dependently (≥ 10 μM) down-regulates the phosphorylation of Akt protein in NSPCs (P<0.01). Meanwhile, p27 expression was increased by ketamine exposure also in a concentration-dependent (≥ 1 μM) manner (P<0.05) (Figure 4A–B). This supports our hypothesis that ketamine inhibits PI3K-Akt signaling pathway, which can release the inhibition of p27 from phosphorylated Akt. The up-regulated p27 protein further promotes cell cycle arrest to inhibit proliferation and enhance differentiation of NSPCs.
Figure 4. Ketamine down-regulates the phosphorylation of Akt and up-regulates the expression of p27.
Adherent monolayer NSPCs cultures were exposed to different concentrations of ketamine (0, 1, 10, 20, 50, and 100 μM). Then, western-blot assays are used to determine the expression of Akt, phosphorylated Akt, p27, and actin in these ketamine treated cultures. Actin was used as an internal scale. The gray density of each band was measured using Image J program. The ratio of the integrated gray density of target proteins to that of corresponding actin represents the quantity of the expression of the target protein. The ratio of p-Akt to Akt represents the quantity of the phosphorylation of Akt proteins. The ratios are plotted in the above statistical chart to show the changing trends. The data are the representative of three independent experiments. Stars represent significant differences compared to the control.
p27 is regulated by the phosphorylation of Akt proteins in NSPCs
To verify that p27 is regulated by PI3K/Akt signaling pathway in NSPCs, we employed PI3K signaling pathway inhibitor LY-294002 to reduce the phosphorylation of Akt. Results show that LY-294002 significantly reduced the phosphorylation of Akt proteins (P<0.01) and up-regulate p27 protein expression (P<0.05). (Figure 5A–B) These results indicate that Akt is an upstream regulator of p27 in NSPCs, which supports the finding that ketamine can also serve an Akt signaling pathway inhibitor, like LY-294002. Thus, ketamine exposure caused cell cycle arrest of proliferating NSPCs can be mediated by up-regulating p27 via inhibiting PI3K/Akt signaling in NSPCs.
Figure 5. p27 is a downstream factor of Akt proteins.

Adherent monolayer NSPCs cultures were exposed to 0 and 10 μM of LY-294002, PI3K signaling pathway inhibitor. Then, western-blot assays are used to determine the expression of phosphorylated Akt, p27, and actin in the cultures. Actin was used as an internal scale. The integrated gray density of each band was measured using Image J program. The ratio of the integrated gray density of target proteins to that of corresponding actin represents the quantity of the expression of the target protein. The ratios are plotted in the above statistical chart. The data are the representative of three independent experiments. Stars represent significant differences compared to the control.
NMDA receptors are involved in ketamine-inhibited PI3K/Akt signaling
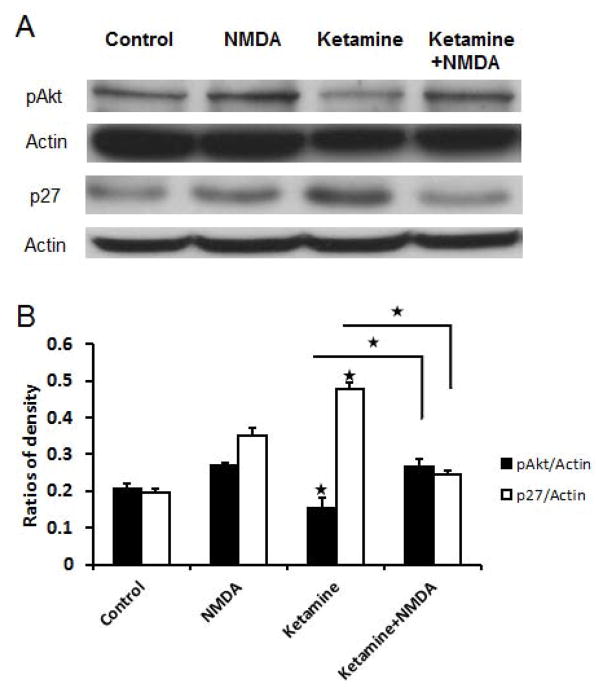
To identify whether ketamine-induced inhibition of the PI3K/Akt signaling pathway was mediated by NMDA receptors, cultured NSPCs were either exposed to no treatment, or NMDA, or ketamine, or NMDA plus ketamine for 24 hours. We found that 10 μM of ketamine reduced phosphorylated Akt (P<0.01) and increased p27 expression (P<0.01) whereas NMDA increased Akt phosphorylation (Figure 6A–B). Compared to the ketamine group (10 μM), phosphorylated Akt protein level was increased (P<0.01) and p27 protein level was decreased in the NMDA plus ketamine group(Figure 6A–B), indicating that NMDA receptor activation can block the inhibitory effects of ketamine on Akt phosphorylation and thereby down-regulate the expression of p27. Taken together, these data suggest that the effects of ketamine on p27 expression through the inhibition of PI3K/Akt signaling are regulated by the activity of NMDA receptors.
Figure 6. NMDA blocks ketamine-induced up-regulation of the expression of p27.
Adherent monolayer NSPCs cultures were treated as control, 50 μM of NMDA, 10 μM of ketamine, and 10 μM of ketamine + 50 μM of NMDA groups. Then, western-blot assays are used to determine the expression of phosphorylated Akt, p27, and Actin in these ketamine treated cultures. Actin was used as an internal scale. The integrated gray density of each band was measured using Image J program. The ratio of the integrated gray density of target proteins to that of corresponding actin represents the quantity of the expression of the target protein. The ratios are plotted in the above statistical chart. The data are the representative of three independent experiments. Stars represent significant differences.
Discussion
In this study, we found that Akt proteins were expressed in neurospheres and adherent monolayer NSPCs, consistent with previous studies(Sinor and Lillien, 2004). BrdU incorporation assays and default differentiation tests showed that the primary isolated and cultured NSPCs have potencies to renew themselves and differentiate into neural specific cells: astrocytes and neurons. Various growth factors, neurotrophic factors, and neurohormones are reported to regulate proliferation and differentiation of NSPCs directly or indirectly, mediated by PI3K/Akt signaling pathway. Our results suggest that this signaling pathway is involved in ketamine-induced changes in NPSCs, mediated by altering the phosphorylation of Akt.
Recent studies support that activation of NMDA receptors regulates the PI3K/Akt signaling pathway. In cultured hippocampal neurons, glutamate concentration-dependently inhibited Akt phosphorylation and these effects were blocked by NMDA receptor antagonists, including MK801 and AP-V, indicating the involvement of the NMDA receptor(van der Heide et al., 2005; Zheng and Quirion, 2009). Our results demonstrate that ketamine concentration-dependently reduced the phosphorylation of Akt in NSPCs, which was blocked by NMDA receptor activation. In ischemic retinal ganglion cells, however, intravitreal administration of the NMDA receptor antagonist, MK801 significantly increased Akt phosphorylation(Russo et al., 2008), whereas other studies show that NMDA does not activate the phosphorylation of Akt(Habas et al., 2006). Thus, ketamine-inhibited phosphorylation of Akt can be regulated by the activity of NMDA receptors, although this inhibition may not be directly mediated by the antagonism of NMDA receptors.
Our recent data show that no calcium influx occurred in NSPCs exposed to ketamine and NMDA in spite of the expression of NMDA receptor subunits NR1 and NR2B, implying the lack of functional NMDA receptors in NSPCs. However, ketamine inhibited the phosphorylation of Akt in a concentration-dependent manner further suggesting that ketamine-induced inhibition of PI3K/Akt signaling pathway may be independent of NMDA receptors. Previous studies provide other potential mechanisms. For example, the SH2 domains of PI3K can interact with tyrosine phosphorylated NR2B subunits of the NMDA receptor(Hisatsume C., 1999) indicating that the NMDA receptor stimulation could directly lead to increases in PKB/Akt phosphorylation. Ketamine may interact with NMDA receptors and reduce the activation of PI3K signaling pathway.
Further experiments demonstrate that p27 is the downstream factor of Akt proteins in NSPCs. The PI3K/Akt signaling pathway inhibitor, LY-294002 dephosphorylates Akt and up-regulates the expression of p27, similar to the concentration-dependent effects of ketamine. Up-regulated p27 increases the inhibition to cdk2 and reduces the proliferation of NSPCs. (Figure 7) This mechanism explains our results from in vivo and in vitro studies, showing that ketamine can inhibit proliferation and enhance neuronal differentiation in cultured NSPCs(Dong et al., 2012) and the developing brain.
Figure 7. Mechanisms of ketamine-altered proliferation and differentiation of NSPCs.

This sketch graph shows potential mechanisms of ketamine-induced changes of the neurogenesis of NSPCs, based on current studies. Ketamine inhibits PI3K/Akt signaling pathway leading to the reduction of phosphorylated Akt proteins by unknown pathways. Reduction of phosphorylated Akt releases the inhibition on p27, a cell cycle inhibitor. Up-regulated p27 can inhibit its downstream factor, cdk2. Inhibited cdk2 can arrest cell cycle and inhibit the proliferation and promotes the differentiation of NSPCs. Dash arrows in the graph represents the effects of ketamine on NSPCs.
Although we did not design experiments to test ketamine relevant cytotoxicity in NSPCs in this study, similar experiments have been done in our previous studies showing that ketamine exposure for 24 hrs (concentrations range: 0, 1, 10, 20, 50, and 100 μM) cannot significantly induce apoptosis and necrosis in in vitro cultured NSPCs(Dong et al., 2012). Therefore, we don’t need worry about the effects of ketamine induced relevant cytotoxicity on the results of this study. However, there are still several limitations of this study. For example, it unknown whether ketamine induced up-regulated NR1 can build up NMDA receptors on cell membrane with NR2B, and whether these NMDA receptors are functional. Clarifying these questions will help us understand the mechanisms of ketamine-induced changes in the neurogenesis of NSPCs. Furthermore, our study is only focused on the PI3K/Akt signaling pathway. This signaling pathway has a broad range of functions on the neurogenesis of NSPCs and can interact with many other signaling pathways regulating the neurogenesis of NSPCs. Therefore, further studies should be designed to confirm the specificity of using PI3K/Akt-p27 signaling pathway to interpret ketamine-induced changes in the neurogenesis of NSPCs.
Ketamine has been widely accepted as an anesthetic, analgesic, or sedative in pediatric clinical settings. Based on previous publication(Sinner and Graf, 2008), a blood level of ketamine for a general anesthesia is 2000–3000 ng/ml, which is equal to around 10.5 μM. Therefore, 10 μM of ketamine was used as a clinically relevant concentration in our in vitro studies which is close to a general dose of ketamine in pediatric practice: 2 mg/kg. Additionally, considering the difference in drug metabolism between human and rodents, a range of ketamine concentrations (0, 1, 10, 20, 50, and 100 μM) was tested in our experiments. In our studies, exposure to 10 μM of ketamine, a clinically relevant concentration, for 24 hours has already induced up-regulations of p27, which can inhibit the proliferation of NSPCs(Dong et al., 2012). The findings suggest that exposure to an anesthesia dose of ketamine in obstetric or pediatric clinical practice can alter proliferation of NSPCs in the developing brain, mediated by inhibiting Akt signaling pathway and up-regulating p27. This study provides a new clue exploring the mechanisms of ketamine-induced developmental neurotoxicity in developing brain.
Acknowledgments
This work was support by the funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) via U10-HD50009, the Oakley Endowed Chair of Critical Care Medicine, Little Rock, AR, St. Jude Chair of Critical Care Medicine, Memphis, TN and the Arkansas Children’s Hospital Student and Clinical Staff Research Grant, Little Rock, AR.
Footnotes
Conflict of Interest
All authors have no conflict of interest to declare.
References
- Brennan P, Mehl AM, Jones M, Rowe M. Phosphatidylinositol 3-kinase is essential for the proliferation of lymphoblastoid cells. Oncogene. 2002;21:1263–1271. doi: 10.1038/sj.onc.1205182. [DOI] [PubMed] [Google Scholar]
- Bruel-Jungerman E, Veyrac A, Dufour F, Horwood J, Laroche S, Davis S. Inhibition of PI3K-Akt signaling blocks exercise-mediated enhancement of adult neurogenesis and synaptic plasticity in the dentate gyrus. PLoS One. 2009;4:e7901. doi: 10.1371/journal.pone.0007901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- Crowder RJ, Freeman RS. Phosphatidylinositol 3-kinase and Akt protein kinase are necessary and sufficient for the survival of nerve growth factor-dependent sympathetic neurons. J Neurosci. 1998;18:2933–2943. doi: 10.1523/JNEUROSCI.18-08-02933.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkers PF, Medema RH, Pals C, Banerji L, Thomas NS, Lam EW, Burgering BM, Raaijmakers JA, Lammers JW, Koenderman L, Coffer PJ. Forkhead transcription factor FKHR-L1 modulates cytokine-dependent transcriptional regulation of p27(KIP1) Mol Cell Biol. 2000;20:9138–9148. doi: 10.1128/mcb.20.24.9138-9148.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C, Rovnaghi CR, Anand KJ. Ketamine alters the neurogenesis of rat cortical neural stem progenitor cells. Critical care medicine. 2012;40:2407–2416. doi: 10.1097/CCM.0b013e318253563c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RA, Kaplan DR, Greenberg ME. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- Gan B, Sahin E, Jiang S, Sanchez-Aguilera A, Scott KL, Chin L, Williams DA, Kwiatkowski DJ, DePinho RA. mTORC1-dependent and -independent regulation of stem cell renewal, differentiation, and mobilization. Proc Natl Acad Sci U S A. 2008;105:19384–19389. doi: 10.1073/pnas.0810584105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas A, Kharebava G, Szatmari E, Hetman M. NMDA neuroprotection against a phosphatidylinositol-3 kinase inhibitor, LY294002 by NR2B-mediated suppression of glycogen synthase kinase-3beta-induced apoptosis. J Neurochem. 2006;96:335–348. doi: 10.1111/j.1471-4159.2005.03543.x. [DOI] [PubMed] [Google Scholar]
- Hisatsume CUH, Mishina M, Yamamoto T. Phosphorylation-dependent interaction of the N-methyl-d-aspartate receptor ε2 subunit with phosphatidylinositol 3-kinase. Genes Cells. 1999;4:657–666. doi: 10.1046/j.1365-2443.1999.00287.x. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–74. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- Kennedy SG, Wagner AJ, Conzen SD, Jordan J, Bellacosa A, Tsichlis PN, Hay N. The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev. 1997;11:701–713. doi: 10.1101/gad.11.6.701. [DOI] [PubMed] [Google Scholar]
- Lei G, Xia Y, Johnson KM. The role of Akt-GSK-3beta signaling and synaptic strength in phencyclidine-induced neurodegeneration. Neuropsychopharmacology. 2008;33:1343–1353. doi: 10.1038/sj.npp.1301511. [DOI] [PubMed] [Google Scholar]
- Liang J, Zubovitz J, Petrocelli T, Kotchetkov R, Connor MK, Han K, Lee JH, Ciarallo S, Catzavelos C, Beniston R, Franssen E, Slingerland JM. PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nat Med. 2002;8:1153–1160. doi: 10.1038/nm761. [DOI] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema RH, Kops GJ, Bos JL, Burgering BM. AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature. 2000;404:782–787. doi: 10.1038/35008115. [DOI] [PubMed] [Google Scholar]
- Murillo H, Huang H, Schmidt LJ, Smith DI, Tindall DJ. Role of PI3K signaling in survival and progression of LNCaP prostate cancer cells to the androgen refractory state. Endocrinology. 2001;142:4795–4805. doi: 10.1210/endo.142.11.8467. [DOI] [PubMed] [Google Scholar]
- Nguyen N, Lee SB, Lee YS, Lee KH, Ahn JY. Neuroprotection by NGF and BDNF against neurotoxin-exerted apoptotic death in neural stem cells are mediated through Trk receptors, activating PI3-kinase and MAPK pathways. Neurochem Res. 2009;34:942–951. doi: 10.1007/s11064-008-9848-9. [DOI] [PubMed] [Google Scholar]
- Paling NR, Wheadon H, Bone HK, Welham MJ. Regulation of embryonic stem cell self-renewal by phosphoinositide 3-kinase-dependent signaling. J Biol Chem. 2004;279:48063–48070. doi: 10.1074/jbc.M406467200. [DOI] [PubMed] [Google Scholar]
- Pap M, Cooper GM. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-Kinase/Akt cell survival pathway. J Biol Chem. 1998;273:19929–19932. doi: 10.1074/jbc.273.32.19929. [DOI] [PubMed] [Google Scholar]
- Park SM, Jung JS, Jang MS, Kang KS, Kang SK. Transforming growth factor-beta1 regulates the fate of cultured spinal cord-derived neural progenitor cells. Cell Prolif. 2008;41:248–264. doi: 10.1111/j.1365-2184.2008.00514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo R, Cavaliere F, Berliocchi L, Nucci C, Gliozzi M, Mazzei C, Tassorelli C, Corasaniti MT, Rotiroti D, Bagetta G, Morrone LA. Modulation of pro-survival and death-associated pathways under retinal ischemia/reperfusion: effects of NMDA receptor blockade. J Neurochem. 2008;107:1347–1357. doi: 10.1111/j.1471-4159.2008.05694.x. [DOI] [PubMed] [Google Scholar]
- Sekimoto T, Fukumoto M, Yoneda Y. 14-3-3 suppresses the nuclear localization of threonine 157-phosphorylated p27(Kip1) EMBO J. 2004;23:1934–1942. doi: 10.1038/sj.emboj.7600198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- Shin I, Yakes FM, Rojo F, Shin NY, Bakin AV, Baselga J, Arteaga CL. PKB/Akt mediates cell-cycle progression by phosphorylation of p27(Kip1) at threonine 157 and modulation of its cellular localization. Nat Med. 2002;8:1145–1152. doi: 10.1038/nm759. [DOI] [PubMed] [Google Scholar]
- Sinner B, Graf BM. Ketamine. Handbook of experimental pharmacology. 2008:313–333. doi: 10.1007/978-3-540-74806-9_15. [DOI] [PubMed] [Google Scholar]
- Sinor AD, Lillien L. Akt-1 expression level regulates CNS precursors. J Neurosci. 2004;24:8531–8541. doi: 10.1523/JNEUROSCI.1470-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Lesche R, Li DM, Liliental J, Zhang H, Gao J, Gavrilova N, Mueller B, Liu X, Wu H. PTEN modulates cell cycle progression and cell survival by regulating phosphatidylinositol 3,4,5,-trisphosphate and Akt/protein kinase B signaling pathway. Proc Natl Acad Sci U S A. 1999;96:6199–6204. doi: 10.1073/pnas.96.11.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton G, Chandler LJ. Activity-dependent NMDA receptor-mediated activation of protein kinase B/Akt in cortical neuronal cultures. J Neurochem. 2002;82:1097–1105. doi: 10.1046/j.1471-4159.2002.01031.x. [DOI] [PubMed] [Google Scholar]
- van der Heide LP, Kamal A, Artola A, Gispen WH, Ramakers GM. Insulin modulates hippocampal activity-dependent synaptic plasticity in a N-methyl-d-aspartate receptor and phosphatidyl-inositol-3-kinase-dependent manner. J Neurochem. 2005;94:1158–1166. doi: 10.1111/j.1471-4159.2005.03269.x. [DOI] [PubMed] [Google Scholar]
- Viglietto G, Motti ML, Bruni P, Melillo RM, D'Alessio A, Califano D, Vinci F, Chiappetta G, Tsichlis P, Bellacosa A, Fusco A, Santoro M. Cytoplasmic relocalization and inhibition of the cyclin-dependent kinase inhibitor p27(Kip1) by PKB/Akt-mediated phosphorylation in breast cancer. Nat Med. 2002;8:1136–1144. doi: 10.1038/nm762. [DOI] [PubMed] [Google Scholar]
- Wang C, Sadovova N, Fu X, Schmued L, Scallet A, Hanig J, Slikker W. The role of the N-methyl-D-aspartate receptor in ketamine-induced apoptosis in rat forebrain culture. Neuroscience. 2005;132:967–977. doi: 10.1016/j.neuroscience.2005.01.053. [DOI] [PubMed] [Google Scholar]
- Wang C, Sadovova N, Hotchkiss C, Fu X, Scallet AC, Patterson TA, Hanig J, Paule MG, Slikker W., Jr Blockade of N-methyl-D-aspartate receptors by ketamine produces loss of postnatal day 3 monkey frontal cortical neurons in culture. Toxicological sciences : an official journal of the Society of Toxicology. 2006;91:192–201. doi: 10.1093/toxsci/kfj144. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Umehara H, Murayama K, Okabe M, Kimura T, Nakano T. Activation of Akt signaling is sufficient to maintain pluripotency in mouse and primate embryonic stem cells. Oncogene. 2006;25:2697–2707. doi: 10.1038/sj.onc.1209307. [DOI] [PubMed] [Google Scholar]
- Xia P, Chen HS, Zhang D, Lipton SA. Memantine preferentially blocks extrasynaptic over synaptic NMDA receptor currents in hippocampal autapses. J Neurosci. 2010;30:11246–11250. doi: 10.1523/JNEUROSCI.2488-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Wang CZ, Liu J, Anastasio NC, Johnson KM. Lithium protection of phencyclidine-induced neurotoxicity in developing brain: the role of phosphatidylinositol-3 kinase/Akt and mitogen-activated protein kinase kinase/extracellular signal-regulated kinase signaling pathways. J Pharmacol Exp Ther. 2008;326:838–848. doi: 10.1124/jpet.107.133272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Xiao Z, Gao Y, Chen B, Zhang J, Dai J. Insulin rescues ES cell-derived neural progenitor cells from apoptosis by differential regulation of Akt and ERK pathways. Neurosci Lett. 2007;429:49–54. doi: 10.1016/j.neulet.2007.09.076. [DOI] [PubMed] [Google Scholar]
- Zheng WH, Quirion R. Glutamate acting on N-methyl-D-aspartate receptors attenuates insulin-like growth factor-1 receptor tyrosine phosphorylation and its survival signaling properties in rat hippocampal neurons. J Biol Chem. 2009;284:855–861. doi: 10.1074/jbc.M807914200. [DOI] [PubMed] [Google Scholar]
- Zou X, Patterson TA, Sadovova N, Twaddle NC, Doerge DR, Zhang X, Fu X, Hanig JP, Paule MG, Slikker W, Wang C. Potential neurotoxicity of ketamine in the developing rat brain. Toxicological sciences : an official journal of the Society of Toxicology. 2009;108:149–158. doi: 10.1093/toxsci/kfn270. [DOI] [PMC free article] [PubMed] [Google Scholar]