Abstract
Accomplishing full, functional integration at the host-to-biomaterial interface has been a critical roadblock in engineering implants with performance similar to biological materials. Molecular recognition-based self-assembly, coupled with biochemical signaling, may lead to controllable and predictable cellular differentiation at the implant interface. Here, we engineer a bio-inspired interface built upon a chimeric peptide. Binding to the biomaterial interface is achieved using a molecular recognition domain specific for the titanium/titanium alloy implant surface and a biochemical signal guiding stem cells to differentiate by activating the Wnt signaling pathway for bone formation. During a critical period of host cell growth and determination, the bioactive implant interface signals mouse, as well as human, stem cells to differentiate along osteogenic lineages. The Wnt-induced cells show enhanced mineral deposition in an extracellular matrix of their creation and an enhanced gene expression profile consistent with osteogenesis, thereby providing a bone-to-implant interface that promotes bone regeneration.
Keywords: Biomaterial Interface, Chimeric Peptide, Wnt signaling, Osteogenesis, Bone Regeneration
Background
Musculoskeletal disease is the second largest cause of disability worldwide (1), with osteoarthritis, the leading cause of hip and knee joint replacement (2), affecting over 30 million people in the USA. Nearly 10% of joint implants fail due to osteolysis and bone defects at the interface (3). Interfacial integrity of the host-implant interface remains a challenge despite significant efforts to improve the currently employed interface fixation approaches (4–10). Attempts to boost implant performance by providing bio-active ligands requiring passive absorption or chemical-coupling have met with limited success due to their non-biological conditions resulting in lost bio-activity (6, 11, 12). Titanium (Ti) and Ti-alloy implants are frequently used for joint replacement and restoration of craniofacial and axial skeletal birth defects, including bone lost to combat injuries. Despite Ti’s biocompatibility, host bone integration crucially depends on the bioactivity of the implant to promote osteointegration.
To address these clinical needs, we engineered a bio-inspired interface for the implant surface, built upon molecular recognition and self-assembly, coupled with the ability to direct cell differentiation by inducing osteogenesis (13–16). A chimeric peptide was constructed from a peptide binding implant (PBI) domain proven effective for specific self-adherence onto an implant surface (17) fused to a bone-inducing protein (BIP) that activates Wnt signaling (16). Wnt is an important regulatory pathway for osteogenic differentiation of mesenchymal stem cells, inducing an increase in osteoblastic transcription factors and extracellular matrix molecules that promote bone biomineralization (18, 19). This approach has the potential to modify the biological response at the interface by binding to the implant surface while harnessing the therapeutic value of the Wnt signaling pathway to induce bone growth.
Methods
The chimeric peptide (PBI-BIP) was synthesized with a 3-mer linker of aminocaproic acid using solid-state chemistry and its identity was confirmed by mass-spectrometry (17). Peptide binding was characterized using fluorescent microscopy and quartzcrystal- microbalance spectrometry. PBI-BIP (0.1 mg/ml; 12 µM) in PBS (pH7.4) was absorbed onto discs of implant material (diameter, 10 mm; thickness, 0.5 mm) by incubation in 48-well plate (total volume: 200 µl) at 37°C under constant agitation for 4 hours. The disc was eluted with PBS (pH7.4) over 28 days at 37°C under constant agitation and released peptides were measured using a NanoDrop spectrophotometer.
Mouse ST2 stromal cells or human bone marrow mesenchymal stem cells (hBMMSCs, Lonza) were cultured on the implant disc for two weeks (ST2) or four weeks (hBMMSCs). Alizarin red S staining was used to quantitate calcium deposited into the extracellular matrix, or the cells were collected and marker gene expression levels quantitated by RNA recovery and conversion to cDNAs followed by real-time PCR using the ΔCt method (16, 20).
Results
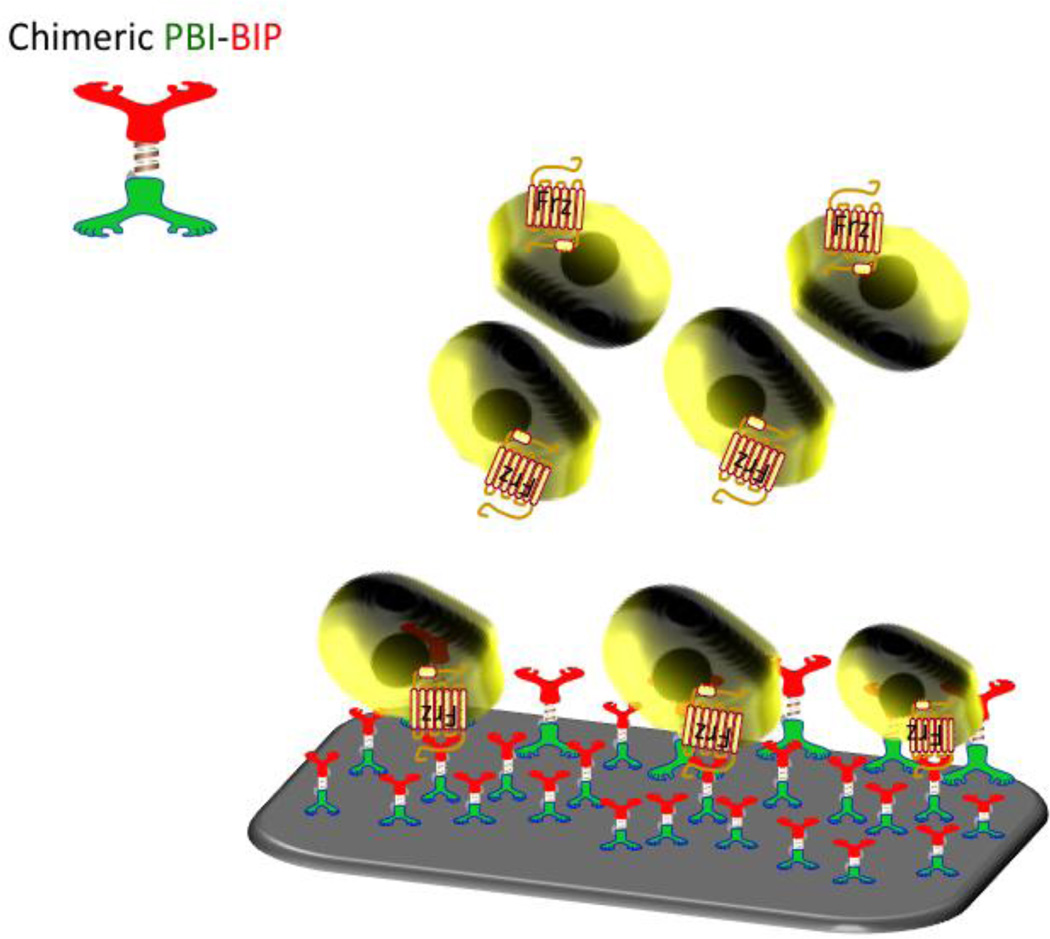
The bio-inspired implant interface, composed of the self-assembling chimeric peptide (PBI-BIP), is shown in Figure 1. Directional binding to the implant surface occurs simultaneously with the display of the Wnt/β-catenin signal that leads to bone formation.
Figure 1. Bio-inspired interface for controlling the interfacial host cell response.
The implant surface is modified by a self-organized chimeric peptide that activates the canonical Wnt beta-catenin signaling pathway of stem cells through the Frizzled receptor leading to regulated gene transcription.
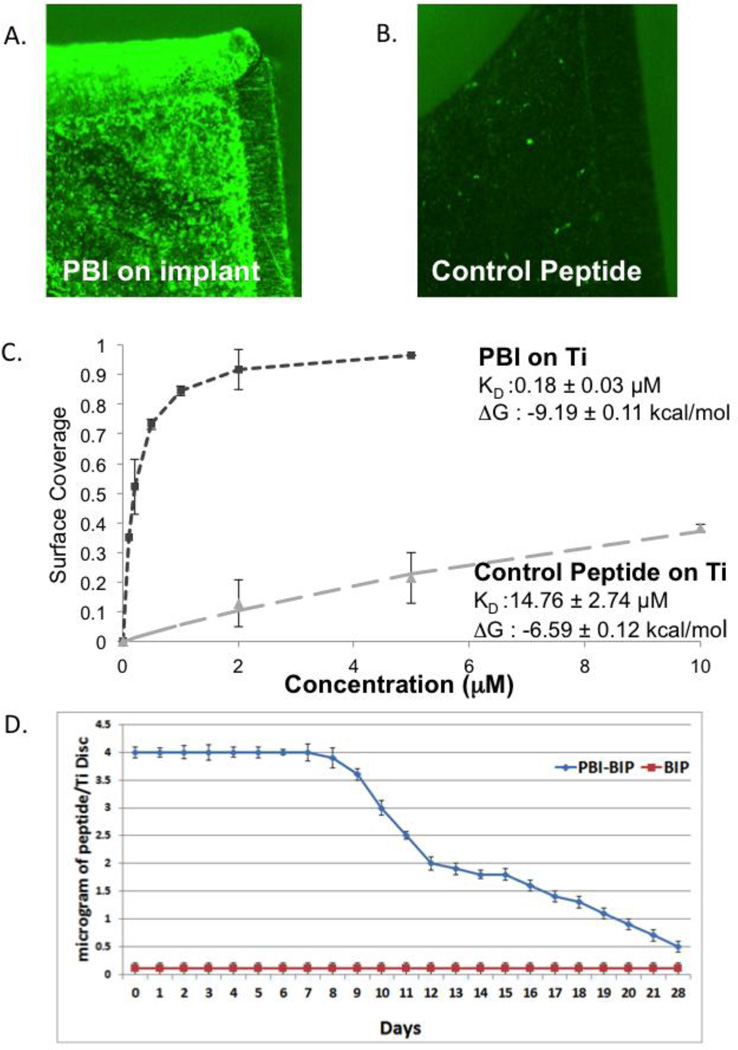
Fluorescent microscopy showed excellent coverage of the implant surface (Figure 2A), whereas the control peptide with its scrambled implant-binding amino acid sequence bound poorly to the implant surface. The PBI exhibited a Kd of 0.18 + 0.03 µM and a ΔG0 of −9.19 + 0.11 Kcal/mol, indicative of its high affinity to the implant surface (Figure 2B). As much as 4 µg of PBI-BIP was bound to the implant disc for 7 days with slow release over the next 21 days, a time frame appropriate in a clinical situation for activation of host cells adherent to the implant by BIP activating Wnt signals (Figure 2C).
Figure 2. Characterization of PBI-BIP binding.
(A) Surface coverage of FITC-labeled peptides on implant surfaces using fluorescence microscopy compared to (B) control peptides; (C) Affinity of PBI-BIP to implant surface; (D) PBI-BIP bound and released from the implant surface.
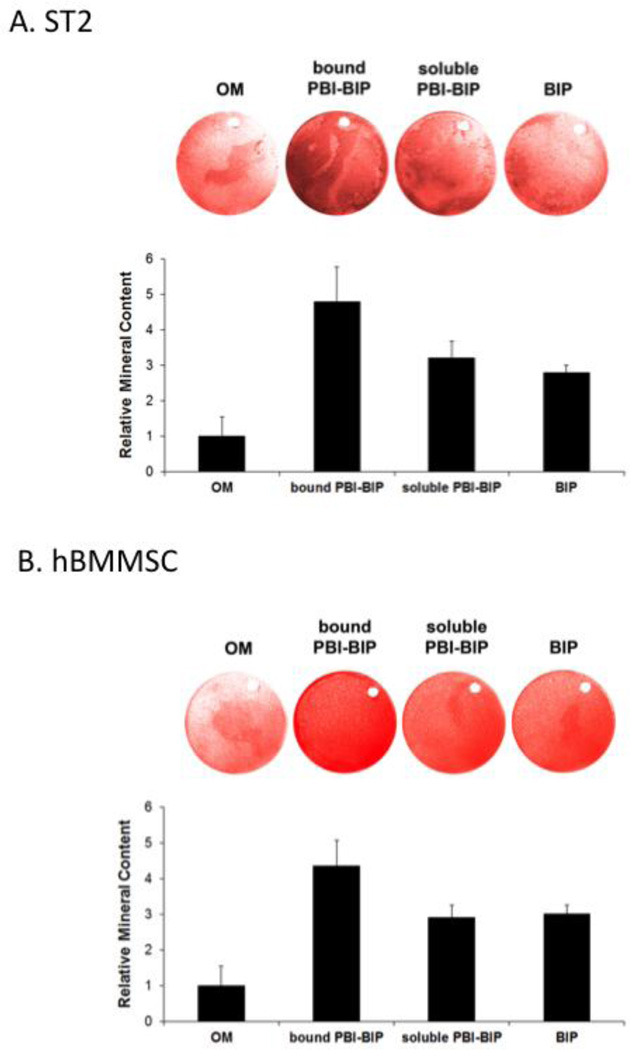
To evaluate the responses of mesenchymal stem cells to PBI-BIP, we employed both a mouse bipotential stromal cell line (ST2) and hBMMSCs. Cells were grown on implant surfaces to over-confluency and induced to osteogenic differentiation for two weeks (ST2) or four weeks (hBMMSCs). Alizarin red S staining identified mineral deposition in the cell-synthesized extracellular matrix. There was a 4.8-fold increase in mineral deposition by ST2 cells (Figure 3A) and a 4.2-fold increase by hBMMSCs (Figure 3B) grown on PBI-BIP bound surfaces, compared to cells grown on bare implant surfaces. Since not all tethered ligands signal appropriately, we corroborated Wnt activation by bound PBI-BIP by independently adding soluble PBI-BIP directly to the osteogenic media. A ~3-fold increase in mineral deposition was observed (Figure. 3), confirming the Wnt activation by the tethered PBI-BIP.
Figure 3. Differentiation of cells grown on PBI-BIP chimeric peptide-bound implant surfaces.
Mouse ST2 cells (A) or hBMMSCs (B) were grown on titanium discs for two weeks (ST2) or four weeks (hBMMSCs). Mineral deposition was assayed with Alizarin Red S staining and quantified. “OM,” osteogenic medium alone; “bound PBI-BIP,” PBI-BIP preloaded onto Ti disc in OM; “soluble PBI-BIP,” PBI-BIP in OM; “BIP,” BIP in OM.
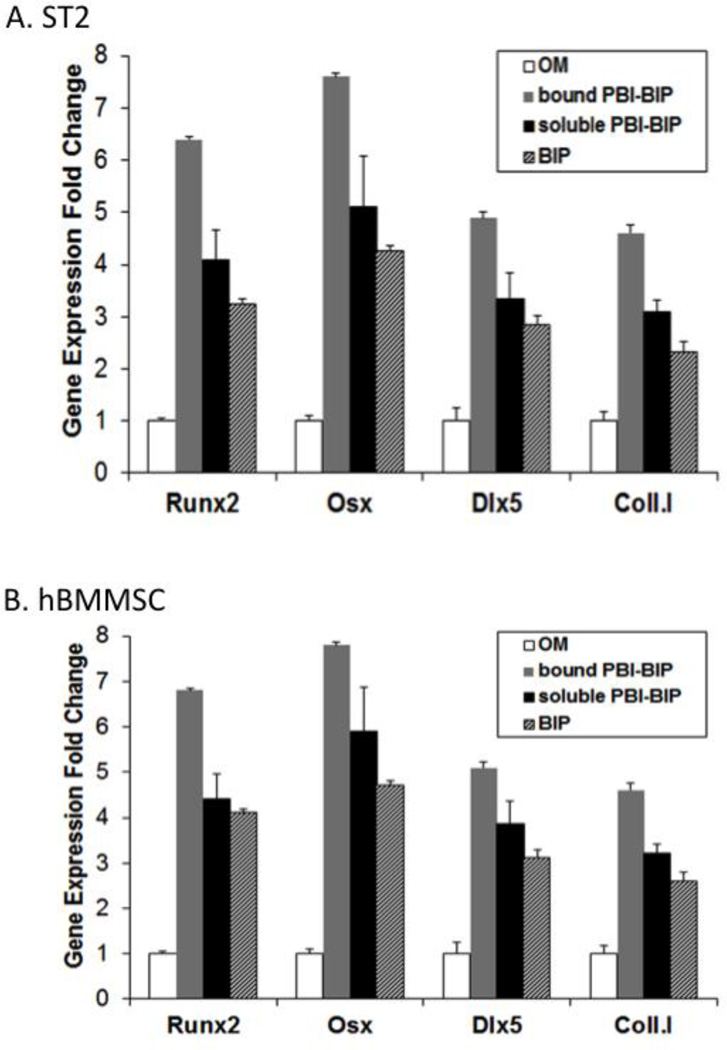
Real-time qRT-PCR analysis was performed for selected osteoblast marker genes (Runx2, Osx, Dlx5 and type I collagen) in ST2 cells grown on implant surfaces treated with PBI-BIP. As expected, the bound chimeric peptide was functional, activating the Wnt signaling pathway for osteogenesis, resulting in a dramatic increase in osteogenic gene expression (Figure 4A).
Figure 4. Bone marker genes in cells grown on PBI-BIP chimeric peptide-bound implant surfaces.
Mouse ST2 cells (A) or hBMMSCs (B) were grown on Ti discs as in figure 3. Osteoblast marker genes were analyzed using qRT-PCR.
Strikingly, hBMMSCs grown on PBI-BIP coated implant surfaces also exhibited guided differentiation (Figure 4B). Bone marker gene analysis demonstrated that a surface of self-organized implant-bound PBI-BIP effectively stimulated osteogenesis of hBMMSCs, suggesting their utility in future clinically relevant studies.
Discussion
In nature, molecular recognition is the key to self-assembling interfaces, as well as to controlling interactions with the surrounding cells through cell surface receptors and downstream signaling pathways that activate specific gene(s) associated with repair and regeneration. Here, cell-to-implant interactions can be directed by a simple one-step procedure using self-organized chimeric peptides. An engineered peptide, PBI-BIP, designed to incorporate implant binding as well as osteogenic activity, was used to guide human stem cells to osteogenesis at the implant interface. This approach is based on molecular recognition and bio-enabled self-assembly, thus avoiding the problems of chemical coupling to the implant interface and the loss of biological activity (17). The modular peptide design permits incorporation of other moieties for cell adhesion or antimicrobial activity to the PBI. Our data demonstrates the potential for tuning the host cell response through an engineered chimeric molecule, which can self-assemble at the implant interface.
An ideal bone replacement construct is expected to functionally replicate the lost tissue while becoming fully integrated with the host bone (10); therefore, materials that simultaneously promote osteogenesis have an added value. Bioengineering the implant surface to enhance bioactivity at the bone-implant interface would be extremely favorable for improving cell-implant interactions and may reduce the lag period before loading can occur. If such interactions can be achieved in animal trials, this approach of modifying the biomaterial interface could shift the tissue-engineering paradigm and improve clinical care.
Supplementary Material
Acknowledgments
Supported by USPHS, NIH, the National Institute of Dental and Craniofacial Research, DE13045 (YZ, MLS), the National Institute of Arthritis and Musculoskeletal and Skin Disease, AR062249 (CT), and the University of Kansas, School of Engineering. We thank Bridget Samuel for excellent assistance with manuscript preparation.
Abbreviations used
- Wnt
int/Wingless family
- Ti
Titanium
- PBI
peptide binding implant
- BIP
bone-inducing protein
- PBI-BIP
chimeric peptide
- ST2
mouse ST2 stromal cells
- hBMMSCs
human bone marrow mesenchymal stem cells
- qRT-PCR
quantitative reverse transcription polymerase chain reaction
- Runx2
Runt-related transcription factor 2
- Osx
osterix
- Dlx5
distal-less homeobox 5
- FITC
fluorescein isothiocyanate
- OM
osteogenic media
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare that there are no commercial associations, current and within the past five years, that might pose a potential, perceived or real conflict of interest.
Bibliography
- 1.Wang HD, Dwyer-Lindgren L, Lofgren KT, Rajaratnam JK, Marcus JR, Levin-Rector A, Levitz CE, Lopez AD, Murray CJL. Age-specific and sex-specific mortality in 187 countries, 1970–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2071–2094. doi: 10.1016/S0140-6736(12)61719-X. PubMed PMID: WOS:000312387000011. [DOI] [PubMed] [Google Scholar]
- 2.Bhatia D, Bejarano T, Novo M. Current interventions in the management of knee osteoarthritis. Journal of pharmacy & bioallied sciences. 2013;5(1):30–38. doi: 10.4103/0975-7406.106561. PubMed PMID: 23559821; PubMed Central PMCID: PMC3612336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith AJ, Dieppe P, Vernon K, Porter M, Blom AW Natl Joint Registry England W. Failure rates of stemmed metal-on-metal hip replacements: analysis of data from the National Joint Registry of England and Wales. Lancet. 2012;379(9822):1199–1204. doi: 10.1016/S0140-6736(12)60353-5. PubMed PMID: WOS:000302230400032. [DOI] [PubMed] [Google Scholar]
- 4.Biomaterials: Important Areas for Future Investment. Biomaterials: Important Areas for Future Investment. NSF Headquarters, Arlington, VA: National Science Foundation; 2012. [Google Scholar]
- 5.Maia FR, Bidarra SJ, Granja PL, Barrias CC. Functionalization of biomaterials with small osteoinductive moieties. Acta biomaterialia. 2013;9(11):8773–8789. doi: 10.1016/j.actbio.2013.08.004. PubMed PMID: 23933486. [DOI] [PubMed] [Google Scholar]
- 6.Cranford SW, de Boer J, van Blitterswijk C, Buehler MJ. Materiomics: An -omics Approach to Biomaterials Research. Advanced Materials. 2013;25(6):802–824. doi: 10.1002/adma.201202553. PubMed PMID: WOS:000314653300001. [DOI] [PubMed] [Google Scholar]
- 7.Saiz E, Zimmermann EA, Lee JS, Wegst UGK, Tomsia AP. Perspectives on the role of nanotechnology in bone tissue engineering. Dental Materials. 2013;29(1):103–115. doi: 10.1016/j.dental.2012.08.001. PubMed PMID: WOS:000312269000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anand R, Graves SE, de Steiger RN, Davidson DC, Ryan P, Miller LN, Cashman K. What is the benefit of introducing new hip and knee prostheses? The Journal of bone and joint surgery American volume. 2011;93(Suppl 3):51–54. doi: 10.2106/JBJS.K.00867. PubMed PMID: MEDLINE:22262424. [DOI] [PubMed] [Google Scholar]
- 9.Dalby MJ, Gadegaard N, Tare R, Andar A, Riehle MO, Herzyk P, Wilkinson CDW, Oreffo ROC. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nature Materials. 2007;6(12):997–1003. doi: 10.1038/nmat2013. PubMed PMID: WOS:000251317000024. [DOI] [PubMed] [Google Scholar]
- 10.Deville S, Saiz E, Tomsia AP. Freeze casting of hydroxyapatite scaffolds for bone tissue engineering. Biomaterials. 2006;27(32):5480–5489. doi: 10.1016/j.biomaterials.2006.06.028. PubMed PMID: WOS:000240794900002. [DOI] [PubMed] [Google Scholar]
- 11.Castner DG, Ratner BD. Biomedical surface science: Foundations to frontiers. Surface Science. 2002;500(1–3):28–60. PubMed PMID: WOS:000175303400003. [Google Scholar]
- 12.Tomsia AP, Launey ME, Lee JS, Mankani MH, Wegst UGK, Saiz E. Nanotechnology Approaches to Improve Dental Implants. International Journal of Oral & Maxillofacial Implants. 2011;26:25–44. PubMed PMID: WOS:000287565800004. [PMC free article] [PubMed] [Google Scholar]
- 13.Levy Y, Onuchic JN. Mechanisms of protein assembly: lessons from minimalist models. Accounts of chemical research. 2006;39(2):135–142. doi: 10.1021/ar040204a. [DOI] [PubMed] [Google Scholar]
- 14.Huang Y, Chiang CY, Lee SK, Gao Y, Hu EL, De Yoreo J, Belcher AM. Programmable assembly of nanoarchitectures using genetically engineered viruses. Nano letters. 2005;5(7):1429–1434. doi: 10.1021/nl050795d. PubMed PMID: 16178252. [DOI] [PubMed] [Google Scholar]
- 15.Evans JS, Samudrala R, Walsh TR, Oren EE, Tamerler C. Molecular design of inorganic-binding polypeptides. Mrs Bulletin. 2008;33(5):514–518. PubMed PMID: WOS:000256032800015. [Google Scholar]
- 16.Wen X, Cawthorn WP, MacDougald OA, Stupp SI, Snead ML, Zhou Y. The influence of Leucine-rich amelogenin peptide on MSC fate by inducing Wnt10b expression. Biomaterials. 2011;32(27):6478–6486. doi: 10.1016/j.biomaterials.2011.05.045. Epub 2011/06/15. PubMed PMID: 21663957; PubMed Central PMCID: PMC3134126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yazici H, Fong H, Wilson B, Oren EE, Amos FA, Zhang H, Evans JS, Snead ML, Sarikaya M, Tamerler C. Biological response on a titanium implant-grade surface functionalized with modular peptides. Acta Biomaterialia. 2013;9(2):5341–5352. doi: 10.1016/j.actbio.2012.11.004. PubMed PMID: WOS:000315170800043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bennett CN, Longo KA, Wright WS, Suva LJ, Lane TF, Hankenson KD, MacDougald OA. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci U S A. 2005;102(9):3324–3329. doi: 10.1073/pnas.0408742102. PubMed PMID: 15728361; PubMed Central PMCID: PMC552924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang S, Bennett CN, Gerin I, Rapp LA, Hankenson KD, Macdougald OA. Wnt signaling stimulates osteoblastogenesis of mesenchymal precursors by suppressing CCAAT/enhancer-binding protein alpha and peroxisome proliferator-activated receptor gamma. J Biol Chem. 2007;282(19):14515–14524. doi: 10.1074/jbc.M700030200. PubMed PMID: 17351296. [DOI] [PubMed] [Google Scholar]
- 20.Warotayanont R, Frenkel B, Snead ML, Zhou Y. Leucine-rich amelogenin peptide induces osteogenesis by activation of the Wnt pathway. Biochem Biophys Res Commun. 2009 doi: 10.1016/j.bbrc.2009.07.058. PubMed PMID: 19615979. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.