Abstract
Increased adiposity and hyperandrogenemia alter reproductive parameters in both animal models and women, but their effects on preantral follicles in the ovary remain unknown. We recently reported that Western-style diet (WSD) consumption over 1 year, with or without chronic exposure to elevated circulating T, increased the body fat percentage, elicited insulin resistance, suppressed estradiol and progesterone production, as well as altered the numbers, size, and dynamics of antral follicles in the ovary during the menstrual cycle in female macaques. Therefore, experiments were designed to compare the WSD and WSD+T effects to age-matched controls on the survival, growth, and function of isolated secondary follicles during 5 weeks of encapsulated 3-dimensional culture. Follicle survival significantly declined in the WSD and WSD+T groups compared with the control (CTRL) group. Although media progesterone levels were comparable among groups, androstenedione and estradiol levels were markedly reduced in the WSD and WSD+T groups compared with the CTRL group at week 5. Anti-Müllerian hormone levels peaked at week 3 and were lower in the WSD+T group compared with the WSD or CTRL group. Vascular endothelial growth factor levels also decreased at week 5 in the WSD+T group compared with the WSD or CTRL group. After human chorionic gonadotropin exposure, only antral follicles developed from the CTRL group yielded metaphase II oocytes. Thus, WSD with or without T exposure affects the cohort of secondary follicles in vivo, suppressing their subsequent survival, production of steroid hormones and local factors, as well as oocyte maturation in vitro.
As recently reviewed by Robker and colleagues (1), female fertility is negatively correlated with increasing body weight (body mass index [BMI], kg/m2), especially obesity (BMI ≥ 30). The infertility associated with obesity is of growing concern, considering the increase in the percent of population that is overweight worldwide, predominantly in developed countries (2). Moreover, a number of studies over the past decade reported detrimental responses in overweight and obese patients to infertility therapy, including i) higher doses of gonadotropin required for ovarian stimulation, ii) decreased numbers of large antral follicles and high-quality oocytes, iii) increased cancellation rates for assisted reproductive technology cycles, iv) greater rates of pregnancy failure, and hence v) reduced live birth rates (3, 4). Studies, particularly in rodent and domestic animal models, are investigating the effect of high levels of lipids (eg, free fatty acids) and adipokines (eg, adiponectin and leptin) on ovarian function (5–7). Collectively, there is mounting evidence that “lipotoxicity” and elevated levels of adipokines can impair follicular development, oocyte quality, and early embryonic development (5, 7). Correlative data from clinical infertility patients support this concept (8). However, to date, research on adipose-ovarian interactions in primates focused primarily on granulosa cells or cumulus-oocyte complexes from antral follicles (9–11), or the relationship between follicular fluid content and fertility outcomes (6, 10). Information regarding the effects of adiposity on preantral follicle development and function is lacking.
Adiposity, and its metabolic or endocrine effects, also reportedly exacerbates various features of polycystic ovarian syndrome (PCOS) (12, 13). A key feature of PCOS is hyperandrogenemia, and changes during obesity may enhance androgen production or action (13). Conversely, androgen excess may influence the level and distribution of adipose tissue (14). Investigators have attempted to evaluate the role of elevated androgens in the etiology and characteristics of PCOS using rodent, domestic animal, and, to a lesser extent, primate models (15–18). However, the combination of hyperandrogenemia with increased adiposity awaits exploration. Also, as noted above, ovarian research has primarily focused on the numerous small-to-medium antral follicles characteristic of PCOS. Information regarding their antecedent preantral follicles is very limited, except for a few studies on primate ovaries analyzing follicles in tissue slices or sections (19, 20), due to a lack of adequate in vitro models.
We performed a pilot study to examine the effects of chronic exposure to elevated T levels, beginning prepubertally (1 year of age), on the hypothalamic-pituitary-ovarian axis and insulin-sensitive glucose metabolism in female macaques (21). Following timely menarche, neuroendocrine effects were noted, with a higher LH response to GnRH and a greater LH pulse frequency during the early follicular phase in T-treated animals at 4 and 5 years of age, respectively, relative to the controls. There were no remarkable differences in insulin sensitivity or antral follicle numbers, but only half the monkeys displayed ovulatory cycles at this age (21). To consider the effects of adiposity and its possible interaction with hyperandrogenemia, these monkeys were switched to a typical Western-style diet (WSD) and experimental parameters monitored for another 18 months (22). As reported recently, not only did the monkeys' percent body fat increase remarkably to 14–19%, the numbers, size, and dynamics of the antral follicle pool also changed, with the level of circulating estradiol (E2) during the follicular phase and diameter of the largest antral follicle diminished in both the WSD and WSD+T groups. Chronic exposure to WSD+T also reduced peak progesterone (P4) levels during the luteal phase and notably decreased insulin sensitivity compared with the WSD group (22). After 18 months of WSD±T, a variety of tissues were collected for additional studies, eg, adipose tissue (23). The ovaries were collected for analysis of tissue sections (24), as well as isolation of preantral and antral follicles for culture and molecular evaluation (25). The current report describes the results of studies examining the survival, growth, and function (production of steroid hormones and paracrine factors, oocyte maturation) of secondary follicles isolated from WSD, WSD+T, and age-matched control monkeys during encapsulated 3-dimensional culture for 5 weeks.
Materials and Methods
Animals and ovary collection
The general care and housing of rhesus macaques was provided by the Division of Comparative Medicine, Oregon National Primate Research Center (ONPRC). Animals were housed in a temperature-controlled (24 ± 2°C) light-regulated (12 h light:12 h dark) room. Animals were treated according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and protocols were approved by the ONPRC Institutional Animal Care and Use Committee (21).
T implants and diet protocol were described in our previous reports (21, 22). Briefly, female rhesus macaques received sc T implants (n = 6) prepubertally beginning at 1 year of age, maintaining a 3.7-fold increase (P = .001) in circulating T levels over cholesterol-implant controls (n = 6) until the end of the study (7 years of age; young adults), based on the clinical evidence that PCOS patients have T levels approximately 3–4 times higher than controls. At 5.5 years, all 12 monkeys were placed on a high-fat, high-fructose diet (WSD), which was maintained for 18 months. Age-matched monkeys (n = 6) on a typical diet (Purina monkey chow supplemented with fresh fruit or vegetables; 26, 27) served as controls. Body weight, percent body fat, and BMI of the monkeys were not different between WSD and WSD+T groups as previously reported (22, 23).
Ovaries were collected from three randomly selected monkeys in each treatment group at their reproductive ages, 7 years for WSD and WSD+T-treated monkeys, 7.3 years for age-matched controls. All nine monkeys exhibited regular menstrual cycles and were evaluated daily for menstruation with the first day of menses termed day 1 of the cycle. Ovariectomies were conducted on anesthetized monkeys by laparoscopy at early follicular phase, day 1–4 of the cycle, as previously described (28). Ovaries were immediately transferred into SAGE OFC Holding Medium (CooperSurgical) at 37°C (26, 27).
Follicle isolation, encapsulation, and culture
Follicle isolation and encapsulation were performed as previously described (26, 27). All procedures were conducted at 37°C. Briefly, ovarian cortex was cut into 1 mm3 cubes. Follicles were mechanically isolated in the Holding Medium using 31-gauge needles. Secondary follicles (diameter, 130–220 μm) that displayed the following characteristics were selected for encapsulation: i) an intact basement membrane, i) healthy granulosa cells, and iii) a visible, healthy oocyte that was round and centrally located within the follicle, without vacuoles or dark cytoplasm. Follicles from each monkey (26 ± 3 follicles/monkey) were assigned for subsequent encapsulation.
Follicles were encapsulated individually into 5-μl alginate matrix containing 0.25% (w/v) sterile sodium alginate (FMC BioPolymers) PBS (137mM NaCl, 10mM phosphate, 2.7mM KCl; Invitrogen), which was crosslinked in 50mM CaCl2, 140mM NaCl, 10mM HEPES solution (pH, 7.2). Each encapsulated follicle was transferred into individual wells of 48-well plates containing 300 μl α-MEM (Invitrogen) supplemented with recombinant human FSH (3 ng/mL from weeks 1–3 and 0.3 ng/mL from weeks 4–5; NV Organon), 0.3% (v/v) human serum protein supplement (CooperSurgical), 5 μg/mL insulin, 0.5 mg/mL purified bovine fetuin, 5 μg/mL transferrin, and 5 ng/mL sodium selenite (Sigma-Aldrich) (26, 27).
Encapsulated follicles were cultured at 37°C in a 5% (v/v) O2 environment (in 6% CO2/89% N2) for 5 weeks. Follicles that reached the antral stage were treated with 100 ng/mL recombinant human chorionic gonadotropin (hCG; Merck Serono) for 34 hours. Oocytes were retrieved to determine their size and stage of meiotic maturation. Half of the culture media (150 μl) was collected and replaced every other day, and stored at −20°C. The media samples from each culture week were assigned to ovarian steroids, anti-Müllerian hormone (AMH), and vascular endothelial growth factor (VEGF) assays (27).
Follicle survival and growth
Follicle survival, diameter, and antrum formation were assessed weekly using an Olympus CK40 inverted microscope and an Olympus DP11 digital camera (Olympus Imaging America) as described previously (26, 27). Follicle photographs were imported into ImageJ 1.42 software (National Institutes of Health) and the diameter of each follicle was measured. Follicles were measured from the outer layer of cells that included a measurement at the widest diameter of the follicle and a second measurement perpendicular to the first. The mean of the values was calculated and reported as the follicle diameter. Follicles were considered to be undergoing atresia if i) the oocyte was dark or not surrounded by a layer of granulosa cells, ii) the granulosa cells became dark and fragmented, or iii) the diameter of the follicle decreased (26).
Follicle histology
Selected in vitro–developed antral follicles were fixed in 4% paraformaldehyde-PBS solution for 3 hours at room temperature. Follicles were embedded in HistoGel (Thermo Scientific) before being dehydrated in ascending concentrations of ethanol (70–100%) and embedded in paraffin. Five micrometer sections were cut by the Imaging and Morphology Support Core at Oregon National Primate Research Center (ONPRC), and stained with hematoxylin and eosin (27).
Ovarian steroids, AMH, and VEGF assays
One media sample collected weekly was analyzed for ovarian steroids by the Endocrine Technology Support Core at ONPRC. P4 and E2 concentrations were assayed using an Immulite 2000, a chemiluminescence-based automatic clinical platform (Siemens Healthcare Diagnostics) validated for macaque follicle culture media (29). Androstenedione (A4) levels were measured by RIA using a DSL-3800 kit (Diagnostic Systems Laboratories) also validated for macaque follicle culture media (30).
Another two media samples collected weekly were analyzed for AMH and VEGF concentrations by ELISA using a DSL-10–14400 kit (Diagnostic Systems Laboratories) and a Human VEGF Quantikine ELISA Kit (R&D Systems), respectively, based on the manufacturers' instructions (31, 32). Both kits were validated for macaque follicle culture media (30, 32). Due to the cross-reaction of fetuin with the AMH antibody, levels assayed in media without cultured follicles were subtracted from AMH levels in media samples as previously described (30).
Oocyte retrieval, maturation, and fertilization
Oocyte retrieval and evaluation were performed on a 37°C warming plate. Cumulus-oocyte complexes were released by breaking the follicle wall using 31-gauge needles in Tyrode's albumin lactate pyruvate (TALP)-HEPES-BSA (0.3%) medium. Cumulus-oocyte complexes were treated with 2 mg/mL hyaluronidase (Sigma-Aldrich) in TALP-HEPES-BSA for 30 seconds to dissociate cumulus cells and obtain denuded oocytes. Retrieved oocytes were transferred to TALP medium and photographed. Oocyte diameters (excluding the zona pellucida) and meiotic status were assessed using the same camera and software as described above (27).
Metaphase II (MII) oocytes were maintained in TALP medium at 37°C in a 20% O2/5% CO2/75% N2 environment for conventional in vitro fertilization (IVF) as previously described (33) within 3 hours of oocyte retrieval. Semen collection was performed by the Assisted Reproductive Technologies (ART) Support Core at ONPRC as previously reported (34). The resulting zygotes were transferred to 500 μl hamster embryo culture medium-9 with 5% fetal bovine serum and cultured at 37°C in 5% O2/6% CO2/89% N2 as previously described (35). Embryos were photographed daily to document development. Reagents and protocols for embryo culture were provided by the Assisted Reproductive Technologies Support Core.
Statistical analysis
Statistical analysis was performed with SAS v9.3 software (SAS Institute). Due to the limited sample size for formal statistical hypothesis testing, a permutation test (randomization test) was used to compare follicle survival rates between groups. A permutation test is a statistical tool that provides statistical inferences tied to chance mechanism in random assignment. Data represent three individual animals in each of the three treatment groups. There were a total of 1680 possible permutations to assign the data to three groups with group size of three. The1680 one-way ANOVAs were performed followed by pair-wise comparison between groups to generate pseudo distributions of groups differences. Follicle growth, steroid/AMH/VEGF production, and oocyte sizes were analyzed for each individual follicle with total follicle numbers indicated in the figure legends, and represent follicles obtained from nine individual animals. Mixed effect model was used with treatment group as between group factor and monkeys nested within the group as random effect. Tukey-Kramer correction for the multiple comparisons was used to correct overall type I error rate. Due to their skewed distribution, the data were transformed using logarithmic function with base 2. Differences were considered significant at P ≤ .05 and values are presented as mean ± SEM.
Results
During manual dissection, we observed that the stroma of ovaries from monkeys with the WSD±T treatment was less compact. The tissue was spongy and clearly atypical wherein follicles were not tightly associated with the stroma compared with ovaries from monkeys fed a typical diet.
Follicle survival
Although their diameters did not increase remarkably during the first 2 weeks of culture, greater than 90% of the secondary follicles from the control group survived, remaining intact with a round oocyte and healthy granulosa cells (Figure 1A). In contrast, greater than 50% of the secondary follicles from the WSD or WSD+T groups degenerated within 2 weeks of culture with a dark and collapsed oocyte (Figure 1A), or oocyte denuded from granulosa cells.
Figure 1.
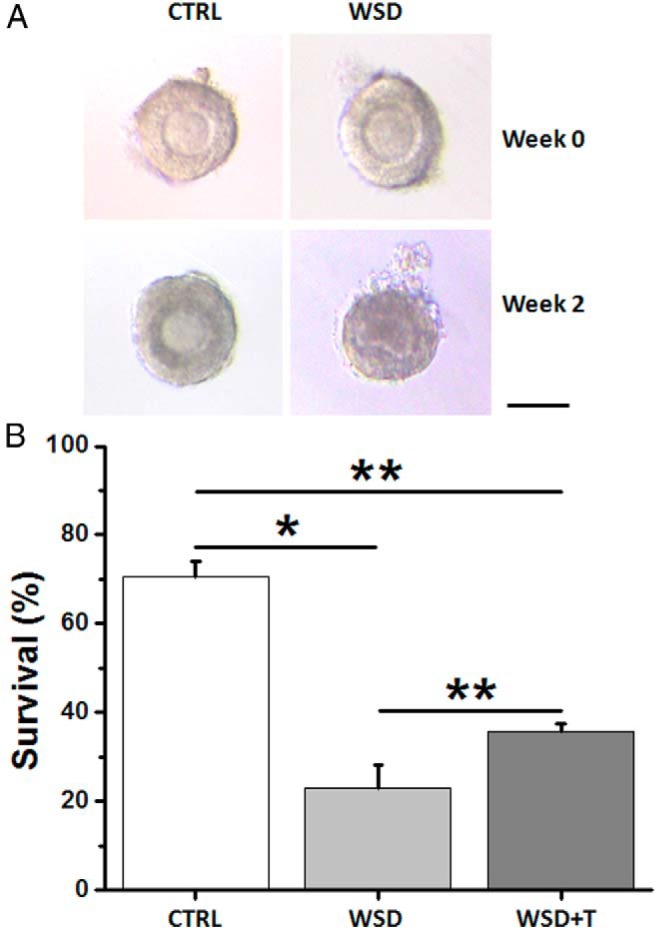
The effects of WSD with and without in vivo T treatment on follicle survival in vitro. A, Follicle morphology at the beginning of culture (week 0) and culture week 2 for the CTRL and WSD groups. Scale bar = 100 μm. B, Follicle survival rate (surviving/total cultured follicles) at week 5 for the CTRL (37/52), WSD (24/108), and WSD+T (27/77) groups. Data are presented as the mean ± SEM with three animals per group. *, significant difference with P < .05; **, significant difference with P < .01.
Follicle survival was markedly lower (P < .05, permutation test) in both the WSD and WSD+T groups compared with controls at week 5 (Figure 1B). However, the WSD+T-treated follicles had a higher (P < .01, permutation test) 5-week survival rate than those of WSD alone (Figure 1B).
Follicle growth and histology
At the beginning of culture, diameters of the surviving follicles did not differ between experimental groups (control vs WSD vs WSD+T = 181 ± 5 vs 180 ± 4 vs 172 ± 5 μm). After 5 weeks, distinct cohorts of surviving follicles were observed based on their growth rate (30). Greater than 80% of surviving follicles from the WSD or WSD+T groups, whereas approximately 40% from the control group, increased their diameters by a minimum of 3-fold (> 500 μm; termed fast-grow follicles) (30). Because there were no differences among the three experimental groups in any other parameters analyzed for the no- or slow-grow follicle cohorts (30; data not shown), only data from the fast-grow follicles are described below.
Diameters increased over the culture period for fast-grow follicles (data not shown), with antrum formation within 3 weeks of culture in all groups (Figure 2A). Diameters of fast-grow follicles were comparable among all experimental groups at week 5 (Figure 2B). Hematoxylin and eosin staining of antral follicles from the control group revealed morphology similar to that observed in primate small antral follicles in vivo (36): a spherical shape with an antrum, a healthy germinal vesicle (GV)-stage oocyte with surrounding cumulus cells, an intact granulosa layer, and presumptive theca cells (Figure 2A). However, follicles in the WSD (Figure 2A) and WSD+T (data not shown) groups had a less-developed granulosa layer surrounded by an extracellular matrix-like structure.
Figure 2.
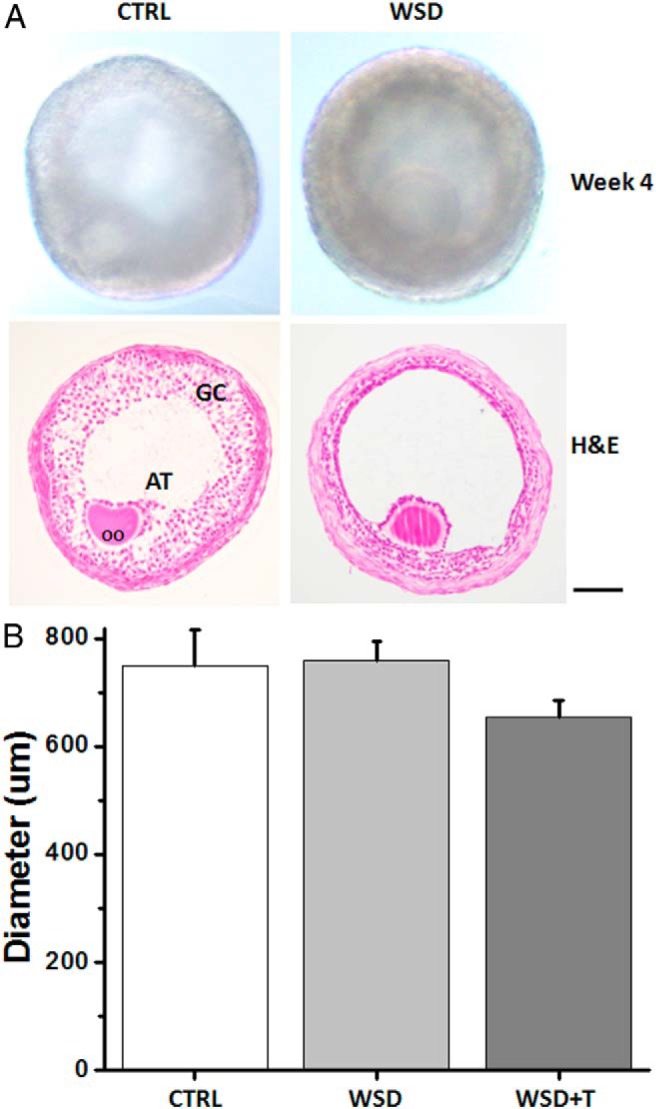
The effects of WSD with and without in vivo T treatment on follicle growth in vitro. A, Morphology of representative small antral follicles at culture week 4 from the CTRL and WSD groups. Scale bar = 100 μm. H&E, hematoxylin and eosin staining; OO, oocyte; AT, antrum; GC, granulosa cells. B, Diameters of fast-grow follicles at week 5 for the CTRL (n = 15), WSD (n = 21), and WSD+T (n = 21) groups. Data are presented as the mean ± SEM with three animals per group.
Follicular steroids
Cultured follicles secreted ovarian steroids into the media beginning at antrum formation with levels increasing through week 5, as observed in our previous studies (26, 27), regardless of the initial in vivo treatment (data not shown). At culture week 5, P4 levels were not different in the WSD or WSD+T groups compared with controls (Figure 3A). In contrast, A4 concentrations produced by fast-grow follicles decreased (P < .05) in the WSD+T, but not WSD, group relative to controls (Figure 3B). Follicles in both the WSD and WSD+T groups produced remarkably lower (P < .01) levels of E2 than controls (Figure. 3C).
Figure 3.
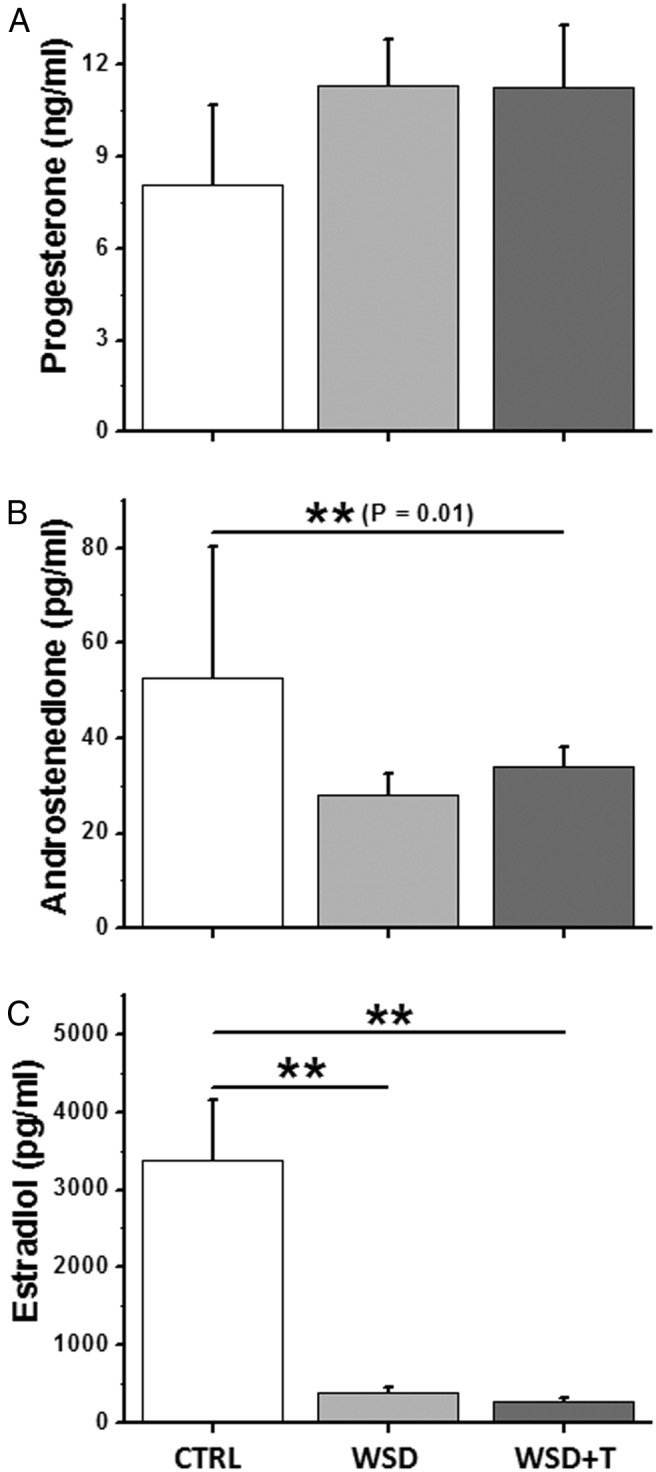
The effects of WSD with and without in vivo T treatment on follicle steroid production in vitro. A, Progesterone; B, androstenedione; and C, estradiol levels produced by fast-grow follicles at week 5 for the CTRL (n = 12), WSD (n = 21), and WSD+T (n = 21) groups. Data are presented as the mean ± SEM with three animals per group. *, significant difference with P < .05; **, significant difference with P < .01.
AMH and VEGF
AMH levels produced by fast-grow follicles were detectable in the media at the beginning of culture and peaked at week 3 after antrum formation, a similar observation to our previous studies (26, 27), in all experimental groups (data not shown). Although there was no difference between the WSD and control groups, WSD+T greatly decreased (P < .01) peak AMH concentrations compared with controls at week 3 (Figure 4A). AMH levels produced by WSD+T-treated follicles at week 3 were also lower (P < .01) than those of WSD follicles.
Figure 4.
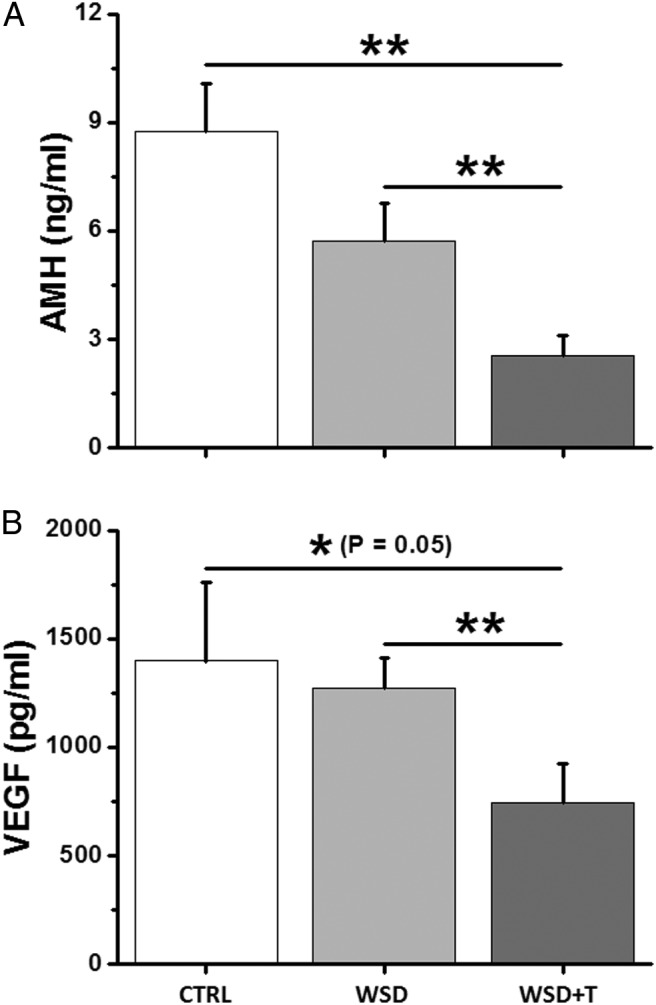
The effects of WSD with and without in vivo T treatment on follicle AMH and VEGF production in vitro. A, Peak AMH levels produced by fast-grow follicles at week 3 for the CTRL (n = 12), WSD (n = 20), and WSD+T (n = 21) groups. B, Peak VEGF levels produced by fast-grow follicles at week 5 for the CTRL (n = 12), WSD (n = 21), and WSD+T (n = 21) groups. Data are presented as the mean ± SEM with three animals per group. *, significant difference with P < .05; **, significant difference with P < .01.
Fast-grow follicles from all experimental groups also produced VEGF, an angiogenic factor generated by follicles after antrum formation, with levels increasing over the 5 weeks of culture (data not shown) as previously reported (27, 30). WSD alone did not alter the VEGF levels at week 5 compared with the controls (Figure 4B). VEGF concentrations produced by WSD+T-treated follicles were lower than those of the WSD (P < .01) and control (P = .05) groups (Figure 4B).
Oocyte diameter, maturation, and fertilization
Following exposure of antral follicles to hCG, healthy, as well as degenerate (dark and condensed cytoplasm), oocytes were retrieved from all experimental groups (Figure 5A). Most of the healthy oocytes remained at the GV stage. Although there was no distinction between the WSD+T and control groups, diameters of GV oocytes were smaller (P < .05) in the WSD group compared with those of the control and WSD+T groups (Figure 5A). MII oocytes were only obtained from the control group with diameters over 120 μm, which were greater than any of the GV oocytes (Figure 5B). After insemination, the MII oocytes fertilized, cleaved, and the embryos developed to the morula stage at day 5 post-IVF (Figure 5C).
Figure 5.
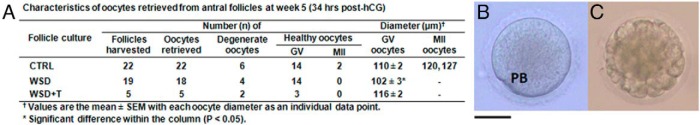
Oocyte retrieval and embryonic development after IVF. A, Characteristics of oocytes retrieved from antral follicles at week 5 from the CTRL, WSD, and WSD + T groups. B, One of two MII oocytes retrieved from the CTRL group. PB, polar body. C, A morula-stage embryo developed in vitro from the inseminated MII oocyte by day 5 post-IVF. Scale bar = 60 μm.
Discussion
We recently developed an encapsulated 3-dimensional culture system that supports the growth of macaque preantral (primary and secondary) follicles to the small antral stage (1–2 mm in diameter), with associated steroid and paracrine factor production, as well as oocyte maturation (26, 27). This in vitro follicle maturation technique allows one to monitor individual follicles and their response to in vitro manipulations (eg, steroid depletion and repletion) (37) or in vivo conditions (eg, stage of the menstrual cycle or aging) (29, 30). This report describes the first study in primates to determine the effects of WSD alone and in combination with chronic exposure to elevated androgen levels in vivo on preantral follicle development in vitro. The data suggest that the WSD, presumably through the diet or increased adiposity (22, 23), negatively affects the cohort of secondary follicles in macaques in terms of their survival and steroidogenic function during culture. Moreover, the addition of chronic hyperandrogenemia, with a 3–4-fold increase in T levels reminiscent of those observed in adolescent girls at risk for PCOS (38), further reduced follicular AMH and VEGF production. Furthermore, such treatments also reduced oocyte growth (diameter, WSD group) and possibly meiotic maturation (WSD and WSD+T groups).
Some alterations in preantral follicles were comparable in both the WSD and WSD+T groups, suggesting a primary effect of diet or adiposity. Whereas secondary follicles from control monkeys typically survived during the first 2 weeks of culture, more than 50% of those in the WSD and WSD+T groups degenerated. We previously reported that macaque secondary follicles require FSH to survive during culture, and fewer follicles survive when cultured in low-dose (10% of current concentration) FSH (26, 30). Given that FSH was present in the culture media, one hypothesis is that the diet/adiposity leads to insufficient FSH receptor (FSHR) expression or signaling in the secondary follicles. Alternatively, the altered hormonal or local milieu caused by diet/adiposity may promote expression of local factors that induce apoptosis and preantral follicle atresia as noted in the rodent ovary (39, 40).
Nevertheless, a subcohort of the secondary follicles survived and grew in a timely manner to small antral follicles at approximately 1 mm diameter by week 5 of culture. However, the ability of these follicles originating from the WSD or WSD+T groups to produce the primary steroid E2 was markedly suppressed. Because P4 levels in the WSD and WSD+T groups did not differ from controls, the defect seems to be in the cytochrome P450 family 17 subfamily A polypeptide 1 and/or 19 subfamily A polypeptide 1 (CYP19A1; aromatase) enzymatic steps. Based on the two-cell model for estrogen production, in which theca cells generate androgen as a precursor (41), insufficient estrogen synthesis could be due to limited development of the theca layer or its function, including cytochrome P450 family 17 subfamily A polypeptide 1expression. Somewhat reduced (40%) A4 levels in the culture media could support such a lesion. However, the reduction in precursor A4 levels was markedly less than that (90%) for E2 levels, suggesting that the primary lesion is in the granulosa cell layer, which results in reduced expression or activity of CYP19A1. Granulosa cell proliferation could be suppressed in the WSD and WSD+T group as suggested by the histologic data. Furthermore, because it is generally accepted that a major action of FSH is to induce CYP19A1 in granulosa cells of the maturing antral follicle (42), one can again hypothesize that a deficit in FSHR or its signaling by adiposity (9, 11), or gonadotropin-regulated paracrine factors (43, 44) leads to the subsequent loss of estrogen synthesis by granulosa cells.
Other alterations in preantral follicles were more pronounced in the WSD+T, compared with the WSD, group, suggesting that elevated androgen alone or acting synergistically with WSD impaired follicular maturation. Notably, AMH and VEGF levels produced by cultured follicles were greatly suppressed by the WSD+T exposure, compared with WSD alone or controls. Our previous culture studies suggested that AMH production increased in growing preantral follicles, and declined or plateaued after antrum formation, whereas VEGF production increased during antral follicle development (27, 30). Thus, T exposure in vivo suppressed the production, and presumably action, of paracrine factors at both the preantral and antral stage in primate follicles in vitro. The decline in AMH levels produced by WSD+T-treated follicles is consistent with previous studies in which obesity (45) and hyperandrogenism (46) are associated with a decreased AMH production by growing follicles. We also noted that FSH promoted AMH and VEGF production by macaque follicles during culture (26, 47). Data from human granulosa cell culture suggested that androgen-receptor signaling increases granulosa cell sensitivity to FSH (48). Therefore, the hypothesized decrease in FSHR signaling and A4 action may limit AMH and VEGF production in WSD+T-exposed follicles.
After secondary follicles from control monkeys develop to the small antral stage in vitro, a small fraction (10–20%) of oocytes reinitiate meiosis and reach the MII stage in response to hCG treatment (26, 27). These oocytes typically achieve a diameter of greater than 115 μm. However, none of the small antral follicles from the WSD or WSD+T groups yielded MII-stage oocytes. This may be related to the inadequate E2 production by WSD- and WSD+T-exposed follicles, which is consistent with the observation that E2 improved the in vitro development of bovine cumulus-oocyte-complex and oocyte nuclear maturation (49). Notably, the mean diameter of the healthy (GV) oocytes from the WSD group was less than that of controls, suggesting that diet or adiposity impaired oocyte growth as noted in obese mice (50). However, diameters of GV oocytes from the WSD+T group were comparable to controls. Thus, androgen levels seem to counteract the effect of WSD on oocyte growth, but still impair their maturation. However, the number of oocytes analyzed from the WSD±T groups was relatively small, due in part to reduced follicle survival. Further investigation is needed on the effects of diet/adiposity with and without hyperandrogenemia on oocyte growth and maturation in primates.
The discovery of altered potential for secondary follicles from WSD and WSD+T groups to survive, grow, and mature in vitro broadens our perspective on the effects of these treatments on primate folliculogenesis. Our prior analyses focused on the antral follicle pool and its steroidogenic potential. Initially, ultrasound analyses determined that WSD±T increased the number of antral follicles but decreased the diameter of the largest antral follicle during the early follicular phase of the menstrual cycle (22). The decreased size of the largest antral follicle (1.0–1.5 mm) was important, because our earlier report noted that a follicle at least 2 mm typically present at or soon after menses was destined to ovulate at midcycle (51). Mean E2 levels during the early follicular phase in WSD and WSD+T animals were also reduced compared with pretreatment values. Ongoing histologic and immunohistochemical studies on sections of excised ovaries (n = 3) removed at the same time as the current studies, confirmed the greater number of antral follicles, primarily due to an increase in degenerating (atretic) antral follicles (24). The current data suggest that defects in the developmental potential of preantral (secondary) follicles precede and portend the dysfunctional antral follicles observed with WSD ± T. Indeed, it is possible that follicles at earlier stages (ie, primordial or primary stages) are also altered by the in vivo milieu (52, 53), leading to dysfunctional secondary and, subsequently, antral follicles. There are limited reports that, although preantral follicles of patients with PCOS seem similar histologically to those of normal women (54), their dynamics in terms of rate of growth or loss may be altered (55). Such alterations in preantral dynamics are likely important for subsequent abnormal antral follicle structure-function.
In conclusion, diet/adiposity seems to modulate preantral (secondary) follicle development and function, as monitored in vitro, which may be exacerbated by chronic hyperandrogenemia. Additional studies are warranted to determine whether treatment-induced deficits in endocrine or paracrine factors and/or their receptor signaling pathways result in impaired follicle survival and reduced steroidogenic function at the subsequent antral stage. Based on other reports, lesions in gonadotropin (particular FSH) or insulin receptor-signaling pathways (56) may be critical. Also, changes in paracrine factor expression or action, including those identified in this study (AMH and VEGF), could be important determinants. The specific features associated with WSD/adiposity, such as lipids, adipokines, inflammatory agents, as well as excess androgen action in vivo, that modify the subsequent developmental potential of secondary follies in vitro await elucidation. The direct effects of steroids and adipokines on macaque follicular development in vitro are under investigation. The current report describes an extended pilot study based on the experimental setting from previous work with limited animal resources (21–23). The findings provide the basis for an ongoing in-depth study (10 monkeys per group), including four animal groups in a 2 × 2 factorial as a function of control vs WSD and with or without chronic T exposure, to discern the effects of WSD/adiposity vs excess androgen exposure in peripubertal to young-adult female monkeys (age 2.5–7.5 y). Our findings may be relevant to understanding the onset of various features of PCOS in overweight adolescent girls and possible treatment.
Acknowledgments
We are grateful to Maralee Lawson, as well as members of the Division of Comparative Medicine, the Endocrine Technology Support Core, the Imaging and Morphology Support Core, the Assisted Reproductive Technologies Support Core, and the Biostatistics Unit, at Oregon National Primate Research Center for their valuable expertise and technical assistance.
This work was supported by the National Institutes of Health Grants UL1DE019587, RL1HD058294, PL1EB008542 (the Oncofertility Consortium), 2K12HD043488 (Building Interdisciplinary Research Careers in Women's Health), RR030276, RR000163, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development through cooperative agreement as part of the Specialized Cooperative Center Program in Reproduction and Infertility Research (Grant No. P50HD071836), and Oregon National Primate Research Center 8P51OD011092.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- A4
- androstenedione
- AMH
- anti-Müllerian hormone
- BMI
- body mass index
- CTRL
- control
- CYP19A1
- cytochrome P450 family 19 subfamily A polypeptide 1
- E2
- estradiol
- FSHR
- FSH receptor
- GV
- germinal vesicle
- hCG
- recombinant human chorionic gonadotropin
- IVF
- in vitro fertilization
- MII
- metaphase II
- ONPRC
- Oregon National Primate Research Center
- P4
- progesterone
- PCOS
- polycystic ovarian syndrome
- TALP
- Tyrode's albumin lactate pyruvate
- VEGF
- vascular endothelial growth factor
- WSD
- Western-style diet.
References
- 1. Wu LL, Norman RJ, Robker RL. The impact of obesity on oocytes: Evidence for lipotoxicity mechanisms. Reprod Fertil Dev. 2011;24:29–34. [DOI] [PubMed] [Google Scholar]
- 2. Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;6736:60460–60468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maheshwari A, Stofberg L, Bhattacharya S. Effect of overweight and obesity on assisted reproductive technology—A systematic review. Hum Reprod Update. 2007;13:433–444. [DOI] [PubMed] [Google Scholar]
- 4. Luke B, Brown MB, Missmer SA, Bukulmez O, Leach R, Stern JE. The effect of increasing obesity on the response to and outcome of assisted reproductive technology: A national study. Fertil Steril. 2011;96:820–825. [DOI] [PubMed] [Google Scholar]
- 5. Mitchell M, Armstrong DT, Robker RL, Norman RJ. Adipokines: Implications for female fertility and obesity. Reproduction. 2005;130:583–597. [DOI] [PubMed] [Google Scholar]
- 6. Yang X, Wu LL, Chura LR, et al. Exposure to lipid-rich follicular fluid is associated with endoplasmic reticulum stress and impaired oocyte maturation in cumulus-oocyte complexes. Fertil Steril. 2012;97:1438–1443. [DOI] [PubMed] [Google Scholar]
- 7. Dupont J, Scaramuzzi RJ, Reverchon M. The effect of nutrition and metabolic status on the development of follicles, oocytes and embryos in ruminants. Animal. 2014;28:1–14. [DOI] [PubMed] [Google Scholar]
- 8. Purcell SH, Moley KH. The impact of obesity on egg quality. J Assist Reprod Genet. 2011;28:517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Greisen S, Ledet T, Møller N, et al. Effects of leptin on basal and FSH stimulated steroidogenesis in human granulosa luteal cells. Acta Obstet Gynecol Scand. 2000;79:931–935. [PubMed] [Google Scholar]
- 10. Robker RL, Akison LK, Bennett BD, et al. Obese women exhibit differences in ovarian metabolites, hormones, and gene expression compared with moderate-weight women. J Clin Endocrinol Metab. 2009;94:1533–1540. [DOI] [PubMed] [Google Scholar]
- 11. Richards JS, Liu Z, Kawai T, et al. Adiponectin and its receptors modulate granulosa cell and cumulus cell functions, fertility, and early embryo development in the mouse and human. Fertil Steril. 2012;98:471–479.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Steckler TL, Herkimer C, Dumesic DA, Padmanabhan V. Developmental programming: Excess weight gain amplifies the effects of prenatal testosterone excess on reproductive cyclicity—Implication for polycystic ovary syndrome. Endocrinology. 2009;150:1456–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barber TM, Franks S. Adipocyte biology in polycystic ovary syndrome. Mol Cell Endocrinol. 2013;373:68–76. [DOI] [PubMed] [Google Scholar]
- 14. Abbott DH, Dumesic DA, Franks S. Developmental origin of polycystic ovary syndrome—A hypothesis. J Endocrinol. 2002;174:1–5. [DOI] [PubMed] [Google Scholar]
- 15. Walters KA, Allan CM, Handelsman DJ. Rodent models for human polycystic ovary syndrome. Biol Reprod. 2012;86:149, 1–12. [DOI] [PubMed] [Google Scholar]
- 16. Abbott DH, Nicol LE, Levine JE, Xu N, Goodarzi MO, Dumesic DA. Nonhuman primate models of polycystic ovary syndrome. Mol Cell Endocrinol. 2013;373:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Padmanabhan V, Veiga-Lopez A. Sheep models of polycystic ovary syndrome phenotype. Mol Cell Endocrinol. 2013;373:8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Houten EL, Visser JA. Mouse models to study polycystic ovary syndrome: A possible link between metabolism and ovarian function? Reprod Biol. 2014;14:32–43. [DOI] [PubMed] [Google Scholar]
- 19. Koering MJ. Preantral follicle development during the menstrual cycle in the Macaca mulatta ovary. Am J Anat. 1983;166:429–443. [DOI] [PubMed] [Google Scholar]
- 20. Vendola KA, Zhou J, Adesanya OO, Weil SJ, Bondy CA. Androgens stimulate early stages of follicular growth in the primate ovary. J Clin Invest. 1998;101:2622–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McGee WK, Bishop CV, Bahar A, et al. Elevated androgens during puberty in female rhesus monkeys lead to increased neuronal drive to the reproductive axis: A possible component of polycystic ovary syndrome. Hum Reprod. 2012;27:531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McGee WK, Bishop CV, Pohl CR, et al. Effects of hyperandrogenemia and increased adiposity on reproductive and metabolic parameters in young adult female monkeys. Am J Physiol Endocrinol Metab. 2014;306:E1292–E1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Varlamov O, Chu MP, McGee WK, et al. Ovarian cycle-specific regulation of adipose tissue lipid storage by testosterone in female nonhuman primates. Endocrinology. 2013;154:4126–4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bishop CV, McGee WK, Galbreath E, Zelinski MB, Cameron JL, Stouffer RL. Western-style diet (WSD) with and without testosterone (T) exposure changes follicular structure-function in young adult, female rhesus monkeys. Fertil Steril. 2013;100:S338. [Google Scholar]
- 25. Xu F, Xu J, Bishop C, Cameron J, Stouffer RL. Transcriptome in small antral follicles (SAFs) of monkeys on a western-style diet (WSD) with/without testosterone (T). Program of the 3rd World Congress of Reproductive Biology, Edinburgh, Scotland, 2014 (Abstract P126). [Google Scholar]
- 26. Xu J, Lawson MS, Yeoman RR, et al. Secondary follicle growth and oocyte maturation during encapsulated three-dimensional culture in rhesus monkeys: Effects of gonadotrophins, oxygen and fetuin. Hum Reprod. 2011;26:1061–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu J, Lawson MS, Yeoman RR, et al. Fibrin promotes development and function of macaque primary follicles during encapsulated three-dimensional culture. Hum Reprod. 2013;28:2187–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Duffy DM, Stouffer RL. Follicular administration of a cyclooxygenase inhibitor can prevent oocyte release without alteration of normal luteal function in rhesus monkeys. Hum Reprod. 2002;17:2825–2831. [DOI] [PubMed] [Google Scholar]
- 29. Xu M, West-Farrell ER, Stouffer RL, Shea LD, Woodruff TK, Zelinski MB. Encapsulated three-dimensional culture supports development of nonhuman primate secondary follicles. Biol Reprod. 2009;81:587–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xu J, Bernuci MP, Lawson MS, et al. Survival, growth, and maturation of secondary follicles from prepubertal, young, and older adult rhesus monkeys during encapsulated three-dimensional culture: Effects of gonadotropins and insulin. Reproduction. 2010;140:685–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fréour T, Mirallié S, Bach-Ngohou K, Denis M, Barrière P, Masson D. Measurement of serum anti-Müllerian hormone by Beckman Coulter ELISA and DSL ELISA: Comparison and relevance in assisted reproduction technology (ART). Clin Chim Acta. 2007;375:162–164. [DOI] [PubMed] [Google Scholar]
- 32. Christenson LK, Stouffer RL. Follicle-stimulating hormone and luteinizing hormone/chorionic gonadotropin stimulation of vascular endothelial growth factor production by macaque granulosa cells from pre- and periovulatory follicles. J Clin Endocrinol Metab. 1997;82:2135–2142. [DOI] [PubMed] [Google Scholar]
- 33. Wolf DP, Vandevoort CA, Meyer-Haas GR, et al. In vitro fertilization and embryo transfer in the rhesus monkey. Biol Reprod. 1989;41:335–346. [DOI] [PubMed] [Google Scholar]
- 34. Lanzendorf SE, Gliessman PM, Archibong AE, Alexander M, Wolf DP. Collection and quality of rhesus monkey semen. Mol Reprod Dev. 1990;25:61–66. [DOI] [PubMed] [Google Scholar]
- 35. Weston AM, Zelinski-Wooten MB, Hutchison JS, Stouffer RL, Wolf DP. Developmental potential of embryos produced by in-vitro fertilization from gonadotrophin-releasing hormone antagonist-treated macaques stimulated with recombinant human follicle stimulating hormone alone or in combination with luteinizing hormone. Hum Reprod. 1996;11:608–613. [DOI] [PubMed] [Google Scholar]
- 36. Gougeon A. Dynamics of human follicular growth: Morphologic, dynamic, and functional aspects. In: Leung PCK, Adashi EY, eds. The Ovary. San Diego, CA: Elsevier Academic Press;2004:25–43. [Google Scholar]
- 37. Rodrigues JK, Navarro PA, Zelinski MB, Stouffer RL, Xu J. Direct actions of androgens on the survival, growth and secretion of steroids and anti-Müllerian hormone by individual macaque follicles during 3-dimentional culture. Hum Reprod. 2015. pii: deu335. [Epub ahead of print] doi: 10.1093/humrep/deu335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Anderson AD, Solorzano CM, McCartney CR. Childhood obesity and its impact on the development of adolescent PCOS. Semin Reprod Med. 2014;32:202–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thompson WE, Asselin E, Branch A, et al. Regulation of prohibitin expression during follicular development and atresia in the mammalian ovary. Biol Reprod. 2004;71:282–290. [DOI] [PubMed] [Google Scholar]
- 40. Belgorosky D, Sander VA, Di Yorio MP, Faletti AG, Motta AB. Hyperandrogenism alters intraovarian parameters during early folliculogenesis in mice. Reprod Biomed Online. 2010;20:797–807. [DOI] [PubMed] [Google Scholar]
- 41. McNatty KP, Makris A, Osathanondh R, Ryan KJ. Effects of luteinizing hormone on steroidogenesis by thecal tissue from human ovarian follicles in vitro. Steroids. 1980;36:53–63. [DOI] [PubMed] [Google Scholar]
- 42. Moon YS, Tsang BK, Simpson C, Armstrong DT. 17 beta-Estradiol biosynthesis in cultured granulosa and thecal cells of human ovarian follicles: Stimulation by follicle-stimulating hormone. J Clin Endocrinol Metab. 1978;47:263–267. [DOI] [PubMed] [Google Scholar]
- 43. Weil S, Vendola K, Zhou J, Bondy CA. Androgen and follicle-stimulating hormone interactions in primate ovarian follicle development. J Clin Endocrinol Metab. 1999;84:2951–2956. [DOI] [PubMed] [Google Scholar]
- 44. Moore RK, Otsuka F, Shimasaki S. Role of ERK1/2 in the differential synthesis of progesterone and estradiol by granulosa cells. Biochem Biophys Res Commun. 2001;289:796–800. [DOI] [PubMed] [Google Scholar]
- 45. Steiner AZ, Stanczyk FZ, Patel S, Edelman A. Antimullerian hormone and obesity: Insights in oral contraceptive users. Contraception. 2010;81:245–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dumesic DA, Patankar MS, Barnett DK, Lesnick TG, Hutcherson BA, Abbott DH. Early prenatal androgenization results in diminished ovarian reserve in adult female rhesus monkeys. Hum Reprod. 2009;24:3188–3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fisher TE, Molskness TA, Villeda A, Zelinski MB, Stouffer RL, Xu J. Vascular endothelial growth factor and angiopoietin production by primate follicles during culture is a function of growth rate, gonadotrophin exposure and oxygen milieu. Hum Reprod. 2013;28:3263–3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nielsen ME, Rasmussen IA, Kristensen SG, et al. In human granulosa cells from small antral follicles, androgen receptor mRNA and androgen levels in follicular fluid correlate with FSH receptor mRNA. Mol Hum Reprod. 2011;17:63–70. [DOI] [PubMed] [Google Scholar]
- 49. Endo M, Kawahara-Miki R, Cao F, et al. Estradiol supports in vitro development of bovine early antral follicles. Reproduction. 2013;145:85–96. [DOI] [PubMed] [Google Scholar]
- 50. Jungheim ES, Schoeller EL, Marquard KL, Louden ED, Schaffer JE, Moley KH. Diet-induced obesity model: Abnormal oocytes and persistent growth abnormalities in the offspring. Endocrinology. 2010;151:4039–4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bishop CV, Sparman ML, Stanley JE, Bahar A, Zelinski MB, Stouffer RL. Evaluation of antral follicle growth in the macaque ovary during the menstrual cycle and controlled ovarian stimulation by high-resolution ultrasonography. Am J Primatol. 2009;71:384–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Koering MJ, Danforth DR, Hodgen GD. Early folliculogenesis in primate ovaries: Testing the role of estrogen. Biol Reprod. 1991;45:890–897. [DOI] [PubMed] [Google Scholar]
- 53. Vendola K, Zhou J, Wang J, Famuyiwa OA, Bievre M, Bondy CA. Androgens promote oocyte insulin-like growth factor I expression and initiation of follicle development in the primate ovary. Biol Reprod. 1999;61:353–357. [DOI] [PubMed] [Google Scholar]
- 54. Chang RJ, Cook-Andersen H. Disordered follicle development. Mol Cell Endocrinol. 2013;373:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Franks S, Stark J, Hardy K. Follicle dynamics and anovulation in polycystic ovary syndrome. Hum Reprod Update. 2008;14:367–378. [DOI] [PubMed] [Google Scholar]
- 56. Vendola K, Zhou J, Wang J, Bondy CA. Androgens promote insulin-like growth factor-I and insulin-like growth factor-I receptor gene expression in the primate ovary. Hum Reprod. 1999;14:2328–2332. [DOI] [PubMed] [Google Scholar]


