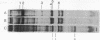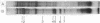Abstract
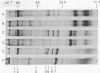
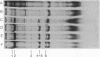
The changes in protein synthesis of salivary glands of Drosophila resulting from a brief exposure at 37 degrees have been analyzed on sodium dodecyl sulfate--acrylamide gels. In D. melanogaster and D. hydei this treatment induces nine and six new puffs, respectively, in the polytene chromosomes. After 20 min treatment seven new proteins are synthesized by the glands of D. melanogaster and six by those of D. hydei as detected by [35S]methionine labeling. Other agents, e.g., recovery from anaerobiosis, induce the same puffs and the same proteins. The extent of protein induction and the degree of puff induction are related to the severity of the temperature treatment. The new proteins are detected after 10 min treatmene at 37 degrees and their synthesis is inhibited by actinomycin D. Actinomycin D added 5 min after the start of temperature treatment has little effect on subsequent protein synthesis. The induced proteins are not tissue specific. Electrophoretic differences of two proteins exist between D. melanogaster and D. simulans, encouraging attempts to map the proteins' gene loci and to test directly whether or not the puffs code for them.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashburner M., Chihara C., Meltzer P., Richards G. Temporal control of puffing activity in polytene chromosomes. Cold Spring Harb Symp Quant Biol. 1974;38:655–662. doi: 10.1101/sqb.1974.038.01.070. [DOI] [PubMed] [Google Scholar]
- Ashburner M. Patterns of puffing activity in the salivary gland chromosomes of Drosophila. V. Responses to environmental treatments. Chromosoma. 1970;31(3):356–376. doi: 10.1007/BF00321231. [DOI] [PubMed] [Google Scholar]
- Ashburner M. Patterns of puffing activity in the salivary gland chromosomes of Drosophila. VI. Induction by ecdysone in salivary glands of D. melanogaster cultured in vitro. Chromosoma. 1972;38(3):255–281. doi: 10.1007/BF00290925. [DOI] [PubMed] [Google Scholar]
- BEERMANN W. Nuclear differentiation and functional morphology of chromosomes. Cold Spring Harb Symp Quant Biol. 1956;21:217–232. doi: 10.1101/sqb.1956.021.01.018. [DOI] [PubMed] [Google Scholar]
- BERENDES H. D., VAN BREUGELF, HOLT T. K. EXPERIMENTAL PUFFS IN SALIVARY GLAND CHROMOSOMES OF DROSOPHILA HYDEI. Chromosoma. 1965 Jan 30;16:35–46. doi: 10.1007/BF00320559. [DOI] [PubMed] [Google Scholar]
- Berendes H. D. Factors involved in the expression of gene activity in polytene chromosomes. Chromosoma. 1968;24(4):418–437. doi: 10.1007/BF00285017. [DOI] [PubMed] [Google Scholar]
- CLEVER U., KARLSON P. [Induction of puff changes in the salivary gland chromosomes of Chironomus tentans by ecdysone]. Exp Cell Res. 1960 Sep;20:623–626. doi: 10.1016/0014-4827(60)90141-5. [DOI] [PubMed] [Google Scholar]
- Ellgaard E. G., Clever U. RNA metabolism during puff induction in Drosophila melanogaster. Chromosoma. 1971;36(1):60–78. doi: 10.1007/BF00326422. [DOI] [PubMed] [Google Scholar]
- Ellgaard E. G. Similarities in chromosomal puffing induced by temperature shocks and dinitrophenol in Drosophila. Chromosoma. 1972;37(4):417–422. doi: 10.1007/BF00284890. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leenders H. J., Beckers P. J. The effect of changes in the respiratory metabolism upon genome activity. A correlation between induced gene activity and an increase in activity of a respiratory enzyme. J Cell Biol. 1972 Nov;55(2):257–265. doi: 10.1083/jcb.55.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leenders H. J., Berendes H. D. The effect of changes in the respiratory metabolism upon genome activity in Drosophila. I. The induction of gene activity. Chromosoma. 1972;37(4):433–444. doi: 10.1007/BF00284892. [DOI] [PubMed] [Google Scholar]
- MacGillivray A. J., Cameron A., Krauze R. J., Rickwood D., Paul J. The non-histone proteins of chromatin, their isolation and composition in a number of tissues. Biochim Biophys Acta. 1972 Aug 25;277(2):384–402. [PubMed] [Google Scholar]
- McKenzie S. L., Henikoff S., Meselson M. Localization of RNA from heat-induced polysomes at puff sites in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1117–1121. doi: 10.1073/pnas.72.3.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poels C. L. Mucopolysaccharide secretion from Drosophila salivary gland cells as a consequence of hormone induced gene activity. Cell Differ. 1972 Jun;1(2):63–78. doi: 10.1016/0045-6039(72)90030-9. [DOI] [PubMed] [Google Scholar]
- SCHOELLMANN G., SHAW E. Direct evidence for the presence of histidine in the active center of chymotrypsin. Biochemistry. 1963 Mar-Apr;2:252–255. doi: 10.1021/bi00902a008. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Tissières A., Mitchell H. K., Tracy U. M. Protein synthesis in salivary glands of Drosophila melanogaster: relation to chromosome puffs. J Mol Biol. 1974 Apr 15;84(3):389–398. doi: 10.1016/0022-2836(74)90447-1. [DOI] [PubMed] [Google Scholar]
- van Breugel F. M. Puff induction in larval salivary gland chromosomes of Drosophila hydei sturtevant. Genetica. 1966;37(1):17–28. doi: 10.1007/BF01547116. [DOI] [PubMed] [Google Scholar]