Abstract
An asymptomatic woman was found to have a compensated hemolytic state due to an unstable hemoglobin variant, comprising 35% of the total. Peptide maps of tryptic digests of the abnormal beta chain were identical to those of beta A except that tryptic peptide 15 (Tyr-His-COOH) was absent and a new peptide was detected, containing equivalent amounts of Ser, Ile, Thr, and Lys. Its sequence was determined by manual Edman degradation. An additional hydrophobic peptide isolated by counter-current distribution contained: Asx, Ser, Ala, Tyr, 2 Phe, and 3 Leu. Its sequence was determined on an automatic solid phase sequencer. Digestion with carboxypeptidase A confirmed the C-terminal sequence. Hemoglobin Cranston has an elongated beta chain with the following structure: (see article). This variant is the first "adult" human hemoglobin known to contain isoleucine. It is likely that hemoglobin Cranston arose because of a nonhomologous crossover of two normal beta chain genes, probably during meiosis, with the insertion of two nucleotides (AG) at position 144, resulting in a frame shift. Hemoglobin Cranston provides new information on the structure of the beta chain gene as well as an explanation of both the structure and genetic basis of hemoglobin Tak, the only other elongated beta chain variant that has been described.
Full text
PDF
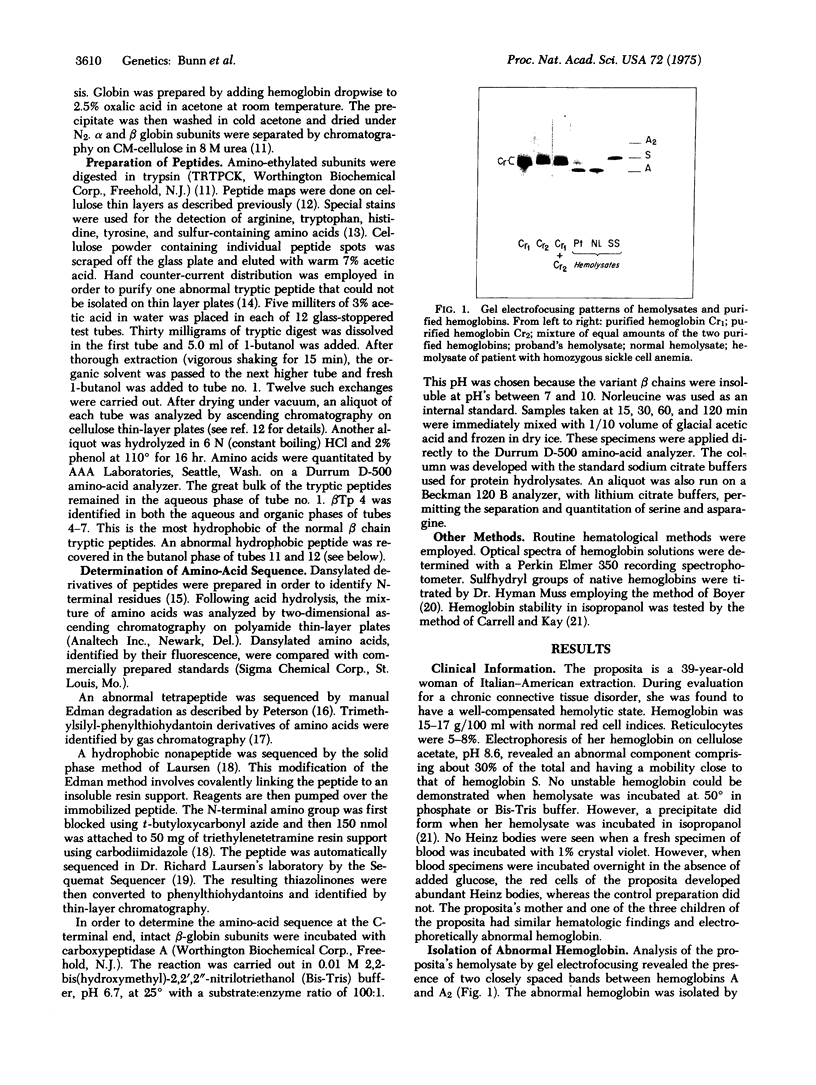
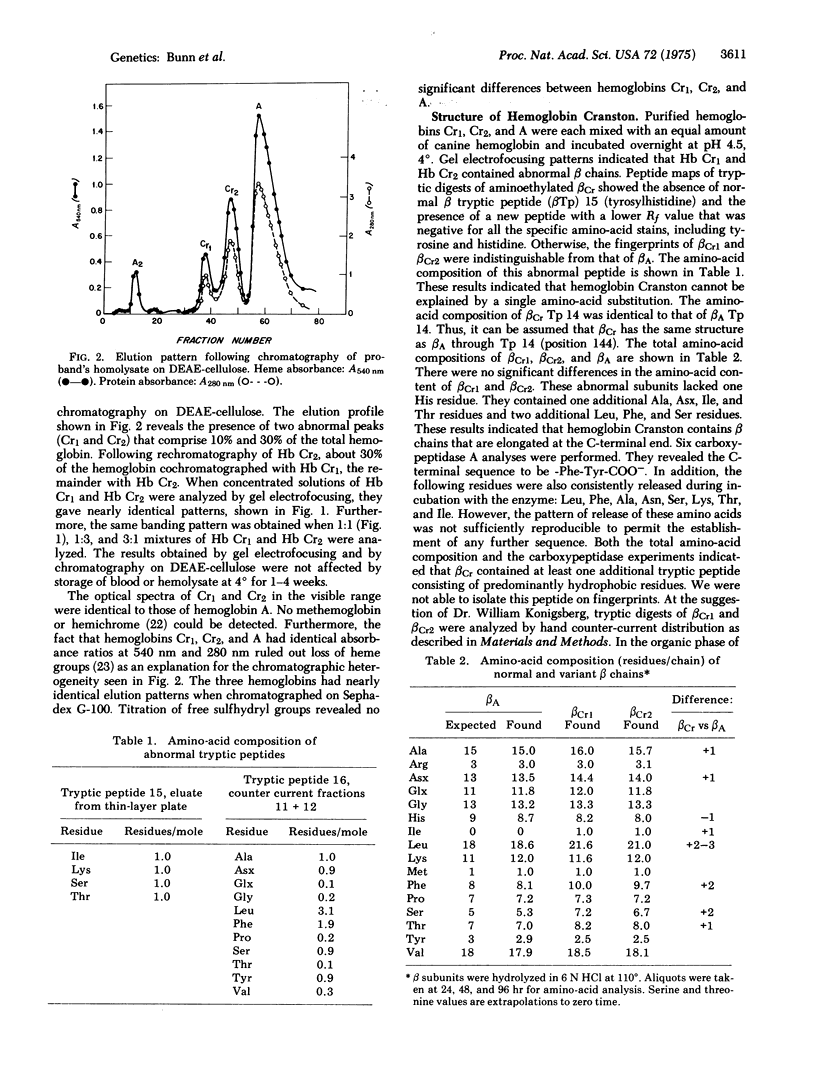


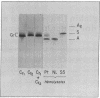
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benz E. J., Jr, Forget B. G. The biosynthesis of hemoglobin. Semin Hematol. 1974 Oct;11(4):463–523. [PubMed] [Google Scholar]
- Carrell R. W., Kay R. A simple method for the detection of unstable haemoglobins. Br J Haematol. 1972 Nov;23(5):615–619. doi: 10.1111/j.1365-2141.1972.tb07096.x. [DOI] [PubMed] [Google Scholar]
- Clegg J. B., Naughton M. A., Weatherball D. J. Abnormal human haemoglobins. Separation and characterization of the alpha and beta chains by chromatography, and the determination of two new variants, hb Chesapeak and hb J (Bangkok). J Mol Biol. 1966 Aug;19(1):91–108. doi: 10.1016/s0022-2836(66)80052-9. [DOI] [PubMed] [Google Scholar]
- Clegg J. B., Weatherall D. J., Contopolou-Griva I., Caroutsos K., Poungouras P., Tsevrenis H. Haemoglobin Icaria, a new chain-termination mutant with causes alpha thalassaemia. Nature. 1974 Sep 20;251(5472):245–247. doi: 10.1038/251245a0. [DOI] [PubMed] [Google Scholar]
- Clegg J. B., Weatherall D. J. Hemoglobin Constant Spring, and unusual alpha-chain variant involved in the etiology of hemoglobin H disease. Ann N Y Acad Sci. 1974;232(0):168–178. doi: 10.1111/j.1749-6632.1974.tb20582.x. [DOI] [PubMed] [Google Scholar]
- Clegg J. B., Weatherall D. J., Milner P. F. Haemoglobin Constant Spring--a chain termination mutant? Nature. 1971 Dec 10;234(5328):337–340. doi: 10.1038/234337a0. [DOI] [PubMed] [Google Scholar]
- De Jong W. W., Meera Khan P., Bernini L. F. Hemoglobin Koya Dora: high frequency of a chain termination mutant. Am J Hum Genet. 1975 Jan;27(1):81–90. [PMC free article] [PubMed] [Google Scholar]
- Drysdale J. W., Righetti P., Bunn H. F. The separation of human and animal hemoglobins by isoelectric focusing in polyacrylamide gel. Biochim Biophys Acta. 1971 Jan 19;229(1):42–50. doi: 10.1016/0005-2795(71)90315-1. [DOI] [PubMed] [Google Scholar]
- Easley C. W. Combinations of specific color reactions useful in the peptide mapping technique. Biochim Biophys Acta. 1965 Sep 13;107(2):386–388. doi: 10.1016/0304-4165(65)90147-9. [DOI] [PubMed] [Google Scholar]
- Fitch W. M. A comparison between evolutionary substitutions and variants in human hemoglobins. Ann N Y Acad Sci. 1974 Nov 29;241(0):439–448. doi: 10.1111/j.1749-6632.1974.tb21900.x. [DOI] [PubMed] [Google Scholar]
- Flatz G., Kinderlerer J. L., Kilmartin J. V., Lehmann H. Haemoglobin Tak: a variant with additional residues at the end of the beta-chains. Lancet. 1971 Apr 10;1(7702):732–733. doi: 10.1016/s0140-6736(71)91994-5. [DOI] [PubMed] [Google Scholar]
- Forget B. G., Marotta C. A., Weissman S. M., Verma I. M., McCaffrey R. P., Baltimore D. Nucleotide sequences of human globin messenger RNA. Ann N Y Acad Sci. 1974 Nov 29;241(0):290–309. doi: 10.1111/j.1749-6632.1974.tb21888.x. [DOI] [PubMed] [Google Scholar]
- Hermodson M. A., Ericsson L. H., Titani K., Neurath H., Walsh K. A. Application of sequenator analyses to the study of proteins. Biochemistry. 1972 Nov 21;11(24):4493–4502. doi: 10.1021/bi00774a011. [DOI] [PubMed] [Google Scholar]
- Jacob H., Winterhalter K. Unstable hemoglobins: the role of heme loss in Heinz body formation. Proc Natl Acad Sci U S A. 1970 Mar;65(3):697–701. doi: 10.1073/pnas.65.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen M., Oski F. A., Nathan D. G., Bunn H. F. Hemoglobin Syracuse (alpha2beta2-143(H21)His leads to Pro), a new high-affinity variant detected by special electrophoretic methods. Observations on the auto-oxidation of normal and variant hemoglobins. J Clin Invest. 1975 Mar;55(3):469–477. doi: 10.1172/JCI107953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING T. P., CRAIG L. C. Countercurrent distribution. Methods Biochem Anal. 1962;10:201–228. doi: 10.1002/9780470110270.ch7. [DOI] [PubMed] [Google Scholar]
- Laursen R. A. Solid-phase Edman degradation. An automatic peptide sequencer. Eur J Biochem. 1971 May 11;20(1):89–102. doi: 10.1111/j.1432-1033.1971.tb01366.x. [DOI] [PubMed] [Google Scholar]
- Marotta C. A., Forget B. G., Weissman S. M., Verma I. M., McCaffrey R. P., Baltimore D. Nucleotide sequences of human globin messenger RNA. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2300–2304. doi: 10.1073/pnas.71.6.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J. D., Nehrlich S., Oyer P. E., Steiner D. F. Determination of the amino acid sequence of the monkey, sheep, and dog proinsulin C-peptides by a semi-micro Edman degradation procedure. J Biol Chem. 1972 Aug 10;247(15):4866–4871. [PubMed] [Google Scholar]
- Rachmilewitz E. A. Denaturation of the normal and abnormal hemoglobin molecule. Semin Hematol. 1974 Oct;11(4):441–462. [PubMed] [Google Scholar]