Abstract
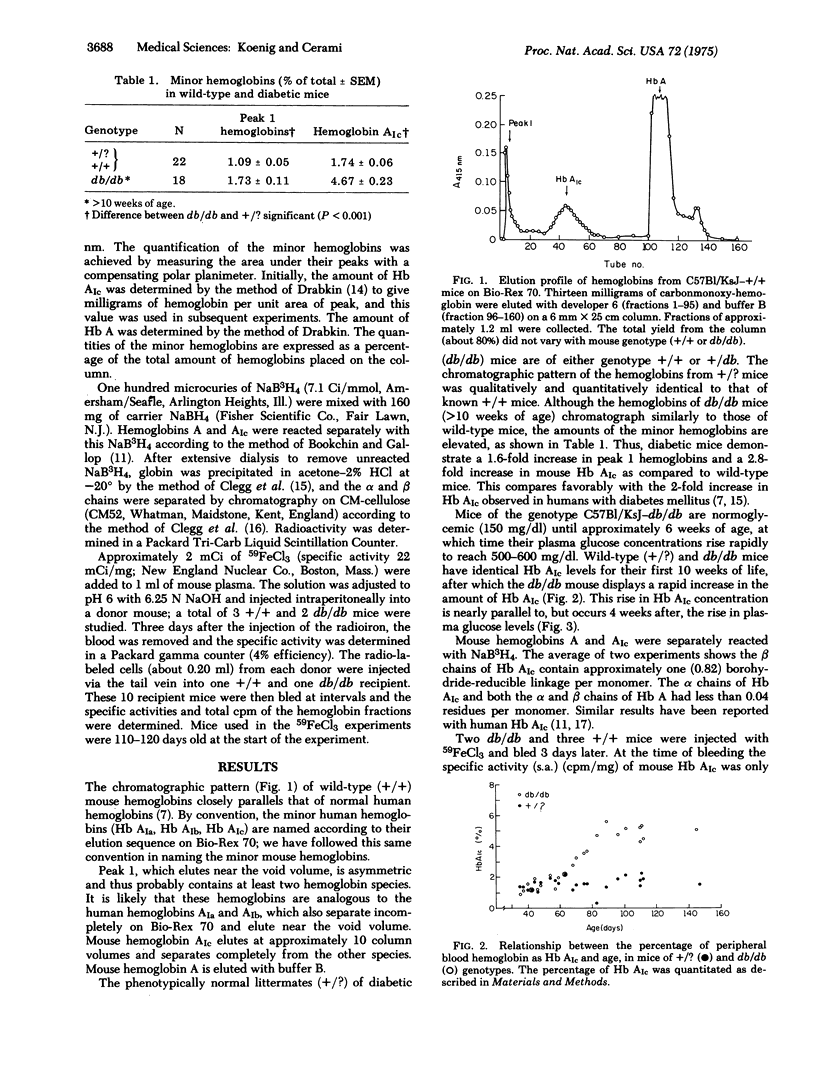
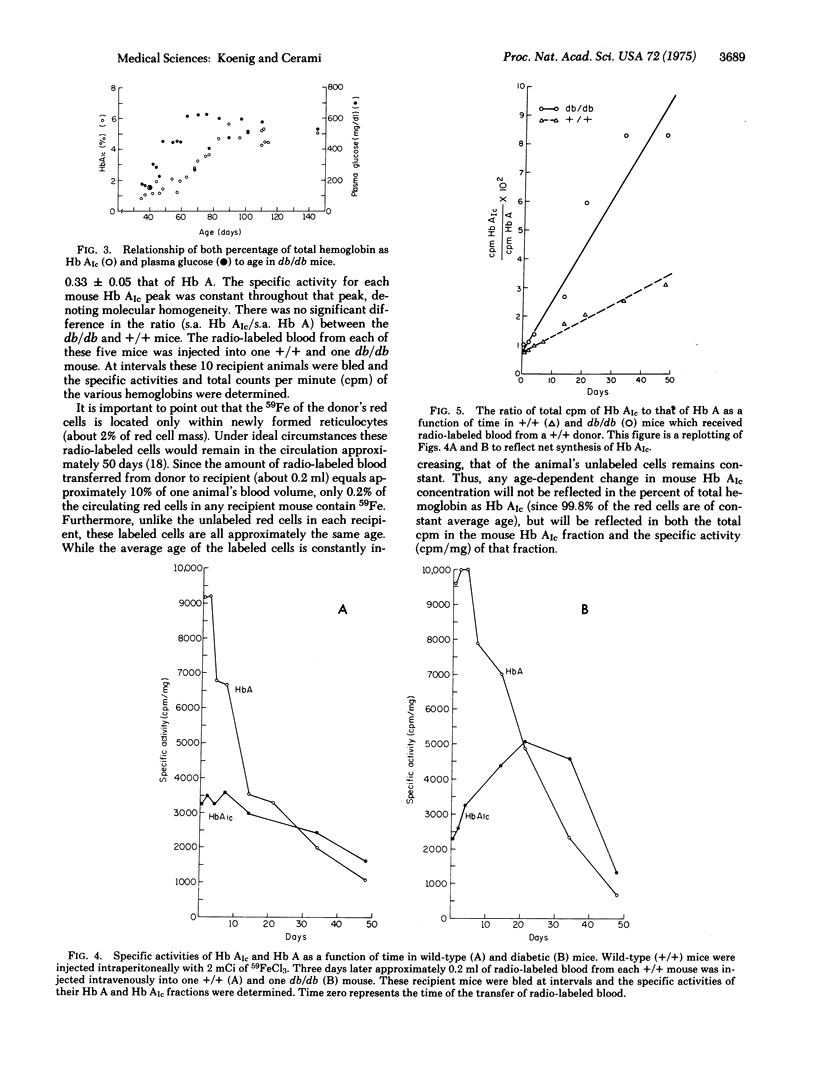
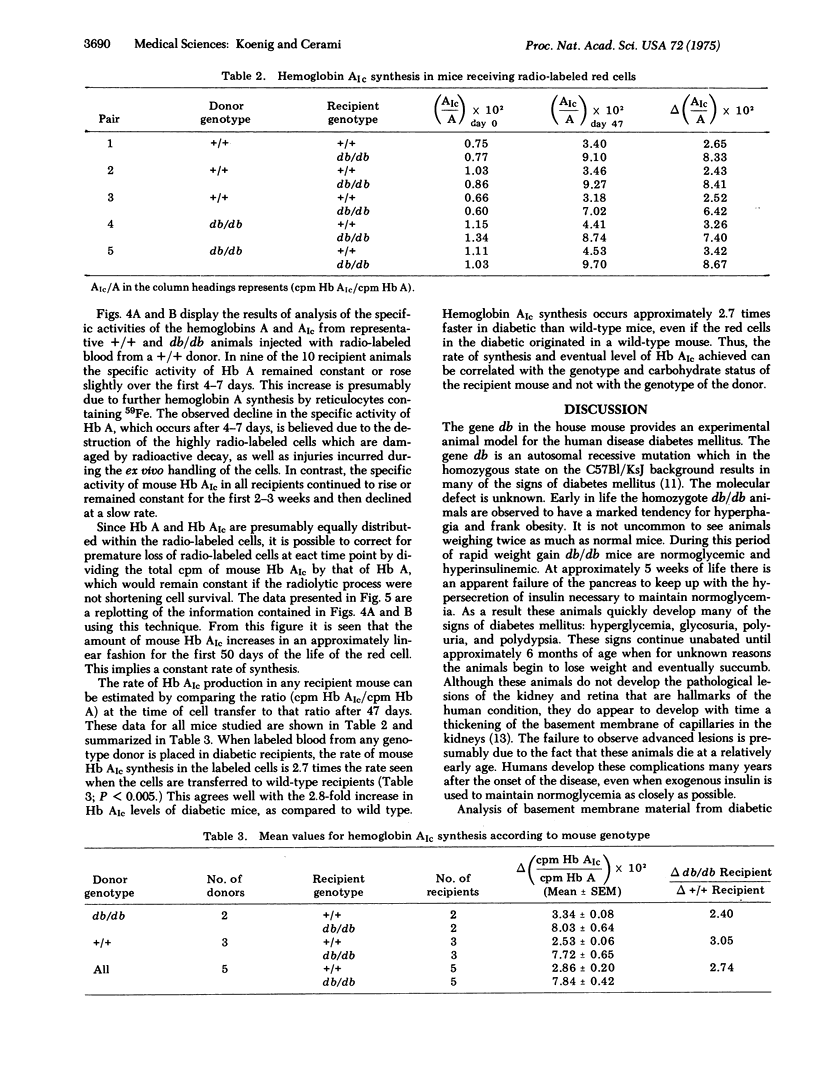
Adult diabetic mice (C57Bl/KsJ--db/db) have increased amounts of a minor hemoglobin in their peripheral blood compared to wild-type (+/+) mice. This increase is analogous to the 2-fold increase of a glycohemoglobin with similar chromatographic mobility (Hb AIc) seen in the blood of patients with diabetes mellitus. Although the exact chemical nature of human or mouse Hb AIc is unknown, both contain a sodium-borohydride-reducible linkage on the beta chain which is a presumed Schiff base between a sugar moiety and the protein. The db/db animals, which have normal amounts of mouse Hb AIc at weaning, show the increase approximately 4 weeks after the onset of the signs of diabetes. This rise is brought about by an increase in a circulating factor that determines directly or indirectly the synthesis of mouse Hb AIc as a post-synthetic modification of Hb A. Evidence for this was obtained by showing that the rate of synthesis of the modified Hb is linear for at least the first 50 days of the life of the red cell and that the rate of synthesis is dependent on the environment in which the cells circulate. Thus the rate of mouse Hb AIc synthesis in +/+ cells is greater when those cells circulate in a db/db host than when they circulate in a +/+ host. The nature of the humoral factor is unknown. If glycosylations of basement membrane proteins and hemoglobin proceed via a common mechanism, then the monitoring of Hb AIc could provide a useful model for studying the early events of basement membrane thickening.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banting F. G., Best C. H., Collip J. B., Campbell W. R., Fletcher A. A. Pancreatic Extracts in the Treatment of Diabetes Mellitus. Can Med Assoc J. 1922 Mar;12(3):141–146. [PMC free article] [PubMed] [Google Scholar]
- Beisswenger P. G., Spiro R. G. Human glomerular basement membrane: chemical alteration in diabetes mellitus. Science. 1970 May 1;168(3931):596–598. doi: 10.1126/science.168.3931.596. [DOI] [PubMed] [Google Scholar]
- Bookchin R. M., Gallop P. M. Structure of hemoglobin AIc: nature of the N-terminal beta chain blocking group. Biochem Biophys Res Commun. 1968 Jul 11;32(1):86–93. doi: 10.1016/0006-291x(68)90430-0. [DOI] [PubMed] [Google Scholar]
- Clegg J. B., Naughton M. A., Weatherall D. J. Separation of the alpha and beta-chains of human hemoglobin. Nature. 1968 Jul 6;219(5149):69–70. doi: 10.1038/219069a0. [DOI] [PubMed] [Google Scholar]
- Clegg J. B., Naughton M. A., Weatherball D. J. Abnormal human haemoglobins. Separation and characterization of the alpha and beta chains by chromatography, and the determination of two new variants, hb Chesapeak and hb J (Bangkok). J Mol Biol. 1966 Aug;19(1):91–108. doi: 10.1016/s0022-2836(66)80052-9. [DOI] [PubMed] [Google Scholar]
- HUISMAN T. H., HORTON B. F. STUDIES ON THE HETEROGENEITY OF HEMOGLOBIN. 8. CHROMATOGRAPHIC AND ELECTROPHORETIC INVESTIGATIONS OF VARIOUS MINOR HEMOGLOBIN FRACTIONS PRESENT IN NORMAL AND IN VITRO MODIFIED RED BLOOD CELL HEMOLYSATES. J Chromatogr. 1965 Apr;18:116–123. doi: 10.1016/s0021-9673(01)80326-5. [DOI] [PubMed] [Google Scholar]
- Holmquist W. R., Schroeder W. A. A new N-terminal blocking group involving a Schiff base in hemoglobin AIc. Biochemistry. 1966 Aug;5(8):2489–2503. doi: 10.1021/bi00872a002. [DOI] [PubMed] [Google Scholar]
- Hummel K. P., Dickie M. M., Coleman D. L. Diabetes, a new mutation in the mouse. Science. 1966 Sep 2;153(3740):1127–1128. doi: 10.1126/science.153.3740.1127. [DOI] [PubMed] [Google Scholar]
- Like A. A., Lavine R. L., Poffenbarger P. L., Chick W. L. Studies in the diabetic mutant mouse. VI. Evolution of glomerular lesions and associated proteinuria. Am J Pathol. 1972 Feb;66(2):193–224. [PMC free article] [PubMed] [Google Scholar]
- Rahbar S., Blumenfeld O., Ranney H. M. Studies of an unusual hemoglobin in patients with diabetes mellitus. Biochem Biophys Res Commun. 1969 Aug 22;36(5):838–843. doi: 10.1016/0006-291x(69)90685-8. [DOI] [PubMed] [Google Scholar]
- Siperstein M. D., Unger R. H., Madison L. L. Studies of muscle capillary basement membranes in normal subjects, diabetic, and prediabetic patients. J Clin Invest. 1968 Sep;47(9):1973–1999. doi: 10.1172/JCI105886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivelli L. A., Ranney H. M., Lai H. T. Hemoglobin components in patients with diabetes mellitus. N Engl J Med. 1971 Feb 18;284(7):353–357. doi: 10.1056/NEJM197102182840703. [DOI] [PubMed] [Google Scholar]
- Vracko R. Skeletal muscle capillaries in diabetics. A quantitative analysis. Circulation. 1970 Feb;41(2):271–283. doi: 10.1161/01.cir.41.2.271. [DOI] [PubMed] [Google Scholar]


