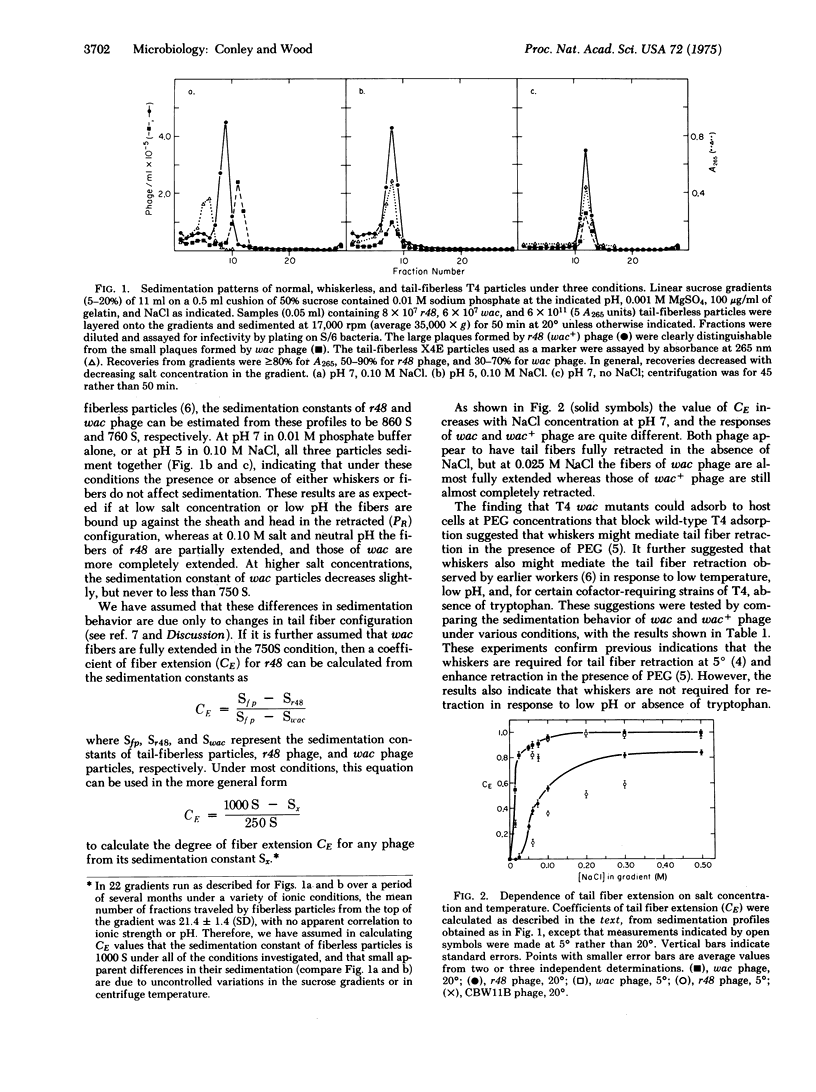
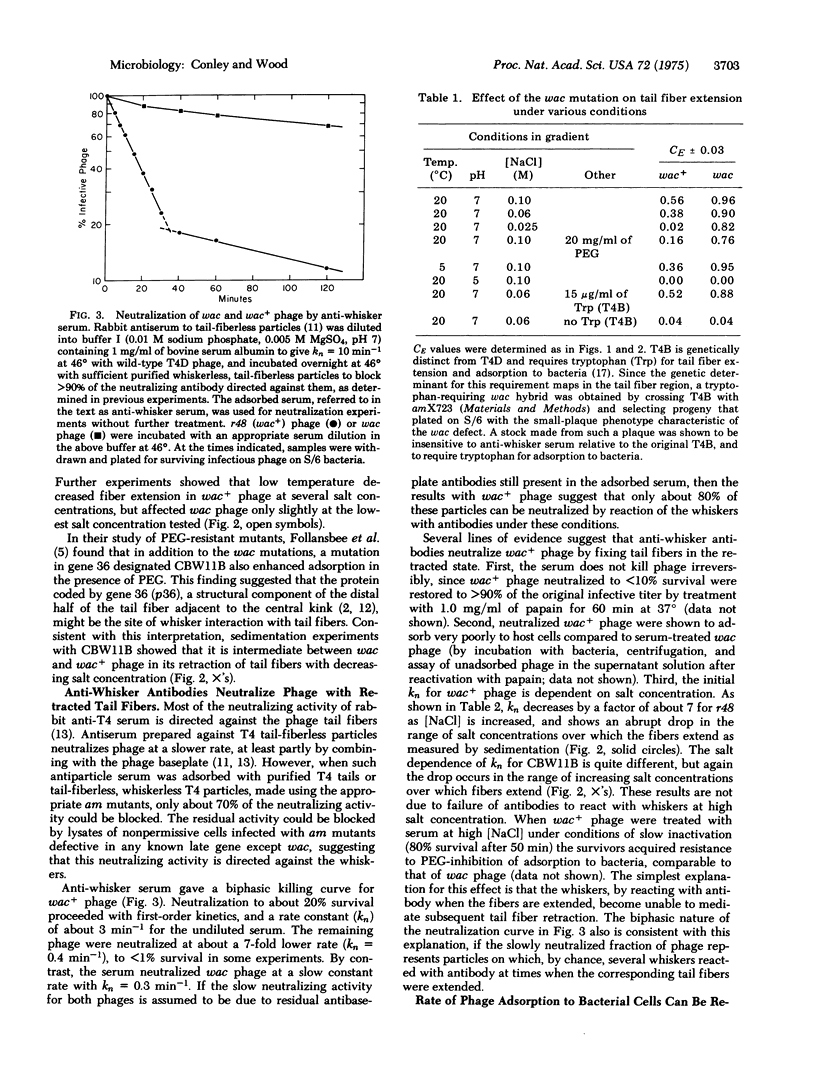
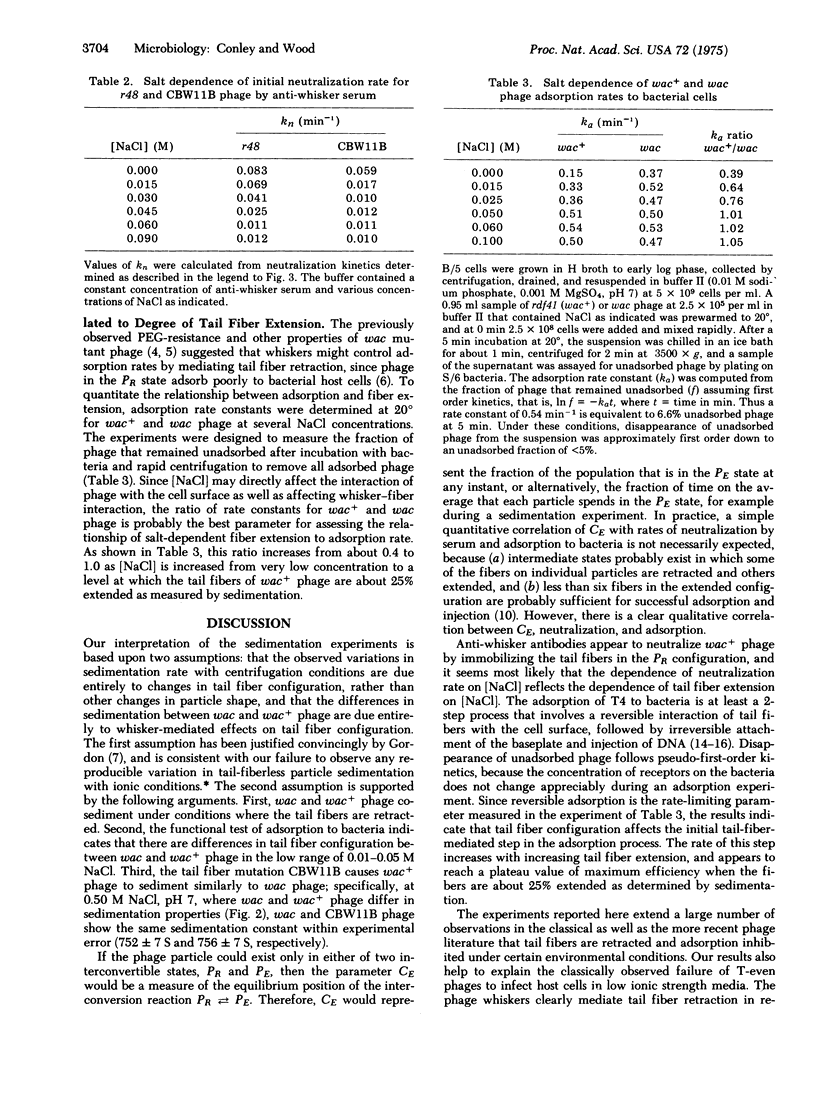
Abstract
The 400 A filaments or "whiskers," which extend outward from the collar region of the phage, control retraction and extension of the tail fibers in response to certain environmental conditions. The tail fibers of normal phage retract in the absence of a required adsorption cofactor, at low pH, at low ionic strength, at low temperature, and at high concentrations of polyethylene glycol. The tail fibers of mutant whiskerless (wac) phage still retract under the first two conditions, but not the last three. Antibodies to whiskers neutralize T4, probably by fixing tail fibers in the retracted configuration. Phage with retracted tail fibers adsorb poorly to host bacterial cells, and their adsorption rate increases as the fibers become extended. These results suggest that one function of the whiskers is to retract the tail fibers and thereby prevent adsorption to host cells under certain conditions that might be unfavorable for production of phage progeny following infection.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRADLEY D. E. The structure of coliphages. J Gen Microbiol. 1963 Jun;31:435–445. doi: 10.1099/00221287-31-3-435. [DOI] [PubMed] [Google Scholar]
- BRENNER S., CHAMPE S. P., STREISINGER G., BARNETT L. On the interaction of adsorption cofactors with bacteriophages T2 and T4. Virology. 1962 May;17:30–39. doi: 10.1016/0042-6822(62)90078-8. [DOI] [PubMed] [Google Scholar]
- Benz W. C., Goldberg E. B. Interactions between modified phage T4 particles and spheroplasts. Virology. 1973 May;53(1):225–235. doi: 10.1016/0042-6822(73)90481-9. [DOI] [PubMed] [Google Scholar]
- Bishop R. J., Conley M. P., Wood W. B. Assembly and attachment of bacteriophage T4 tail fibers. J Supramol Struct. 1974;2(2-4):196–201. doi: 10.1002/jss.400020214. [DOI] [PubMed] [Google Scholar]
- Cummings D. J., Chapman V. A., DeLong S. S. The sedimentation and conformational variance among T-even bacteriophages. Virology. 1969 Jan;37(1):94–108. doi: 10.1016/0042-6822(69)90310-9. [DOI] [PubMed] [Google Scholar]
- Dewey M. J., Wiberg J. S., Frankel F. R. Genetic control of whisker antigen of bacteriophage T4D. J Mol Biol. 1974 Apr 25;84(4):625–634. doi: 10.1016/0022-2836(74)90120-x. [DOI] [PubMed] [Google Scholar]
- Dickson R. C. Assembly of bacteriophage T4 tail fibers. IV. Subunit composition of tail fibers and fiber precursors. J Mol Biol. 1973 Oct 5;79(4):633–647. doi: 10.1016/0022-2836(73)90068-5. [DOI] [PubMed] [Google Scholar]
- Dickson R. C., Barnes S. L., Eiserling F. A. Structural proteins of bacteriophage T4. J Mol Biol. 1970 Nov 14;53(3):461–474. doi: 10.1016/0022-2836(70)90077-x. [DOI] [PubMed] [Google Scholar]
- Edgar R. S., Lielausis I. Serological studies with mutants of phage T4D defective in genes determining tail fiber structure. Genetics. 1965 Dec;52(6):1187–1200. doi: 10.1093/genetics/52.6.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follansbee S. E., Vanderslice R. W., Chavez L. G., Yegian C. D. A new set of adsorption mutants of bacteriophage T4D: identification of a new gene. Virology. 1974 Mar;58(1):180–199. doi: 10.1016/0042-6822(74)90153-6. [DOI] [PubMed] [Google Scholar]
- Gamow R. I., Kozloff L. M. Chemically induced cofactor requirement for bacteriopage T4D. J Virol. 1968 May;2(5):480–487. doi: 10.1128/jvi.2.5.480-487.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon C. N. Dimensions of the heads of the fast and slow sedimenting forms of bacteriophage T2L. J Mol Biol. 1972 Apr 14;65(3):435–445. doi: 10.1016/0022-2836(72)90200-8. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., BOLLE A., BOYDELATOUR E., EPSTEIN R. H., FRANKLIN N. C., JERNE N. K., REALE SCAFATI A., SECHAUD J. FUNCTIONS AND PROPERTIES RELATED TO THE TAIL FIBERS OF BACTERIOPHAGE T4. Virology. 1965 Jul;26:419–440. doi: 10.1016/0042-6822(65)90006-1. [DOI] [PubMed] [Google Scholar]
- King J. Assembly of the tail of bacteriophage T4. J Mol Biol. 1968 Mar 14;32(2):231–262. doi: 10.1016/0022-2836(68)90007-7. [DOI] [PubMed] [Google Scholar]
- STEINBERG C. M., EDGAR R. S. A critical test of a current theory of genetic recombination in bacteriophage. Genetics. 1962 Feb;47:187–208. doi: 10.1093/genetics/47.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon L. D., Anderson T. F. The infection of Escherichia coli by T2 and T4 bacteriophages as seen in the electron microscope. I. Attachment and penetration. Virology. 1967 Jun;32(2):279–297. doi: 10.1016/0042-6822(67)90277-2. [DOI] [PubMed] [Google Scholar]
- Simon L. D., Anderson T. F. The infection of Escherichia coli by T2 and T4 bacteriophages as seen in the electron microscope. II. Structure and function of the baseplate. Virology. 1967 Jun;32(2):298–305. doi: 10.1016/0042-6822(67)90278-4. [DOI] [PubMed] [Google Scholar]
- Wood W. B., Henninger M. Attachment of tail fibers in bacteriophage T4 assembly: some properties of the reaction in vitro and its genetic control. J Mol Biol. 1969 Feb 14;39(3):603–618. doi: 10.1016/0022-2836(69)90148-x. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Uchida H. Organization and function of bacteriophage T4 tail. I. Isolation of heat-sensitive T4 tail mutants. Virology. 1973 Mar;52(1):234–245. doi: 10.1016/0042-6822(73)90412-1. [DOI] [PubMed] [Google Scholar]
- Yanagida M., Ahmad-Zadeh C. Determination of gene product positions in bacteriophage T4 by specific antibody association. J Mol Biol. 1970 Jul 28;51(2):411–421. doi: 10.1016/0022-2836(70)90151-8. [DOI] [PubMed] [Google Scholar]


