Significance
Juvenile hormone (JH) plays key roles in insect development, reproduction, and many other physiological functions. Because JH is specific to insects, it has been investigated for use as pest control. Although compounds that mimic the action of JH (JH analogues/agonists) are efficient, they have a limited scope of application. Development of potent compounds counteracting JH action (JH antagonists) would find a wider range of control applications. However, thus far, such JH antagonists have not been developed. Here, we report on the discovery of potent JH antagonists in plants, which represents an innate resistance mechanism of plants against insect herbivores. These newly discovered plant JH antagonist compounds could be used as the starting material for developing novel insecticides.
Keywords: juvenile hormone, receptor, endocrine disruptor
Abstract
Insects impact human health through vector-borne diseases and cause major economic losses by damaging crops and stored agricultural products. Insect-specific growth regulators represent attractive control agents because of their safety to the environment and humans. We identified plant compounds that serve as juvenile hormone antagonists (PJHANs). Using the yeast two-hybrid system transformed with the mosquito JH receptor as a reporter system, we demonstrate that PJHANs affect the JH receptor, methoprene-tolerant (Met), by disrupting its complex with CYCLE or FISC, formation of which is required for mediating JH action. We isolated five diterpene secondary metabolites with JH antagonist activity from two plants: Lindera erythrocarpa and Solidago serotina. They are effective in causing mortality of mosquito larvae at relatively low LD50 values. Topical application of two diterpenes caused reduction in the expression of Met target genes and retardation of follicle development in mosquito ovaries. Hence, the newly discovered PJHANs may lead to development of a new class of safe and effective pesticides.
Insects cause enormous economic damage and human suffering. Diseases transmitted by insects result in a million deaths per year (1), and insect infestation leads to annual losses of agricultural products worth billions of US dollars (2). The high toxicity of currently available insecticides presents environmental and health risks, and growing resistance and cross-resistance of insects to these existing insecticides gravely complicates the situation. Hence, there is an urgent need to develop novel effective insecticides.
Insect growth regulators (IGRs) have been devised based on insect-specific functions. Three major classes of IGRs are commercially available (3). These IGRs include juvenile hormone (JH) agonists (methoprene and pyriproxyfen), ecdysone agonists (halofenozide and tebufenozide), and chitin synthase inhibitors (buprofezine). They possess low off-target toxicity and environmental danger and have been used to control pests. JH-based IGRs are of particular interest because JH is an insect-specific hormone. JH agonists (JHAs) disrupt insect endocrine regulation, causing abnormal development and larval fatality (4). However, JHAs have limitations in the scope of their applications because they mimic and enhance the JH mode of action. Theoretically, JH antagonists (JHANs) could be used as a powerful alternative, but no effective JHANs have yet been developed.
Recent studies identified methoprene-tolerant (Met) as the JH receptor (5, 6). Met is a member of the family of basic helix–loop–helix (bHLH)-Per-Arnt-Sim (PAS) transcription factors that requires homo- or heterodimerization for DNA binding and transcriptional regulation (7). In the Aedes aegypti mosquito, Met forms a heterodimer with other bHLH-PAS factors, the steroid receptor coactivator (SRC/FISC), or Cycle (CYC) in a JH-dependent manner (8, 9). JH-mediated Met-CYC binding has been quantitatively simulated using the two-hybrid yeast β-galactosidase assay in a yeast cell, Y187 (9). Thus, it is possible to test both JHAs and JHANs for their interactions with Met and its partners using this assay.
Previously, Balsam fir juvabione has been found to inhibit the metamorphosis of the linden bug, Pyrrhocoris apterus, suggesting that plants could use JHAs as resistance factors against insect herbivores (10, 11). However, screening of plants for JH activity revealed only a few JHAs (12). We hypothesized that JHANs, rather than JHAs, play a significant role in plant defense against insect herbivores. In this study, we observed that plants produce JHANs as secondary metabolites. Through two-hybrid yeast β-galactosidase assay screening of 1,651 plant species, we identified 53 species that exhibited putative JHAN activity based on mosquito larvicidal toxicity tests. We isolated five diterpene secondary metabolites with JHAN activity from two plants: Lindera erythrocarpa and Solidago serotina. They were effective at causing mortality of mosquito larvae at relatively low LD50 values. Moreover, topical application of two of these five diterpenes caused reduction in the expression of Met target genes and the retardation of follicle development in mosquito ovaries.
Results and Discussion
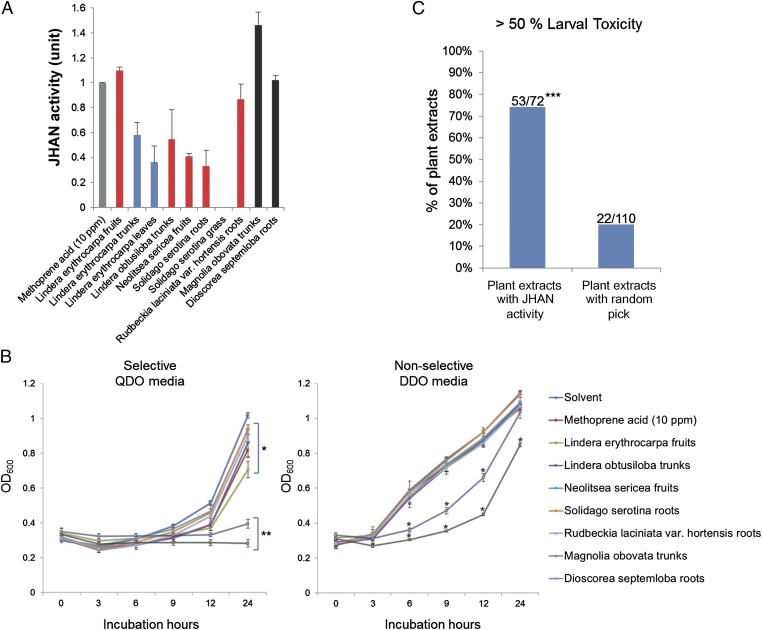
The cDNAs encoding full ORFs of Met and CYC genes from two mosquitoes, A. aegypti and Culex pipiens, and a storage crop pest beetle, Tribolium castaneum, were synthesized and introduced into yeast two-hybrid bait and prey plasmids, respectively. In this two-hybrid yeast assay, binding of Met and CYC from the mosquitoes occurred in the presence of a JHA, pyriproxyfen, whereas that from the beetle did not (Fig. S1). Instead, we observed pyriproxyfen-mediated binding between the beetle Met and SRC (Fig. S1). Although the pyriproxyfen-mediated binding of A. aegypti Met-CYC, C. pipiens Met-CYC, and the beetle Met-SRC was apparent in Y2HGold yeast cells (Fig. S1), only the binding between A. aegypti Met-CYC was successfully simulated by two-hybrid yeast β-galactosidase assay in Y187 yeast cells (Fig. S2). The weak β-galactosidase activity observed in the binding of C. pipiens Met-CYC and the beetle Met-SRC does not necessarily indicate weak affinity of the binding itself, because the affinity could be affected by many additional arbitrary factors during the simulation, such as folding of recombinant proteins. Therefore, we used A. aegypti Met-CYC for all subsequent experiments. First, we investigated whether the pyriproxyfen-mediated Met-CYC binding could be disrupted by plant extracts in the two-hybrid yeast assay. We tested methanol extracts prepared from 1,651 plant species (Korean Plant Extracts Bank, Daejeon, Korea), directly adding each extract to the yeast culture. Extracts from 83 plants were found to interfere with pyriproxyfen-mediated binding of A. aegypti Met-CYC. Fig. 1A shows a few examples. In a parallel study, we tested the effect of methoprene acid (MA) on the pyriproxyfen-mediated Met-CYC binding in the two-hybrid yeast assay. Because MA interfered with the binding in a dose-dependent manner, it was used as a positive control during screening (Figs. 1A and 2B).
Fig. 1.
Screening of the plant extracts harboring JHAN and mosquito larvicidal activity. (A) JHAN activity of some plant extracts. The Met-CYC binding triggered by 33 ng/Ml pyriproxyfen was simulated by β-galactosidase activity in the yeast two-hybrid system. Inhibition of β-galactosidase activity by treatment with 10 ppm methoprene acid was used as a positive control. Each plant extract was added to the yeast culture at a concentration of 100 ppm. When multiple extracts from the same plant species were screened to have JHAN activity, only the extract with the strongest activity was selected for further screening (red column). For example, the trunks and leaves of L. erythrocarpa were excluded from screening (blue column) because the extract from the plant fruits had the strongest JHAN activity. (B) Distinction between JHAN and anti-yeast activity. Two plant extracts from Magnolia obovata trunks and Dioscorea septemloba roots (A, black column) were excluded from the screening because they significantly inhibited yeast growth compared with the control solvent. *P < 0.01 (Student t test), **P < 1.0E-5 (Student t test). (C) Correlation between JHAN activity and larvicidal toxicity in the screened plant extracts. ***P < 0.0001 (two-sample z-test).
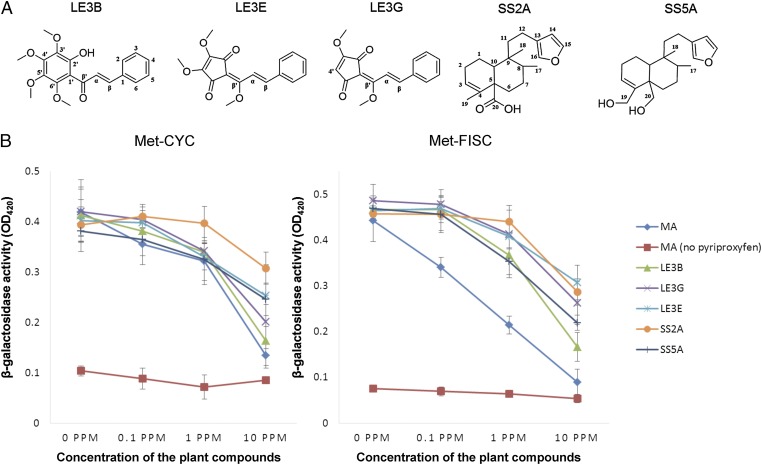
Fig. 2.
PJHAN compounds and their JHAN activities. (A) Chemical structures of five PJHAN diterpene molecules isolated from Lindera erythrocarpa fruits and Solidago serotina roots identified by 1H–NMR, 13C-NMR, and HRESIMS. (B) Concentration-dependent inhibition of pyriproxyfen-mediated Met-CYC or Met-FISC binding by the isolated diterpenes. To initiate the binding, 0.033 ppm of pyriproxyfen was applied into each reaction.
To eliminate the possibility of false signals originating from anti-yeast activity of plant extracts, we tested growth inhibition of the Y2HGold yeast strain transformed with Met and CYC (see Fig. 1B for examples). The addition of several plant extracts interfered with Met-CYC binding, resulting in normal yeast growth in nonselective double dropout minimal media (DDO, -leu/-trp), whereas retarded growth was recorded in selective quadruple dropout minimal media (QDO, -leu/-trp/-his/-ade; Fig. 1B). The plant extracts that caused yeast growth retardation in both DDO and QDO media, such as extracts from Magnolia obovata trunks and Dioscorea septemloba roots, were eliminated from further screening (Fig. 1 A and B). Among 83 extracts from plant species that were initially screened, 72 passed this test but 11 failed due to their inhibitory action on yeast growth. Hence, we concluded that these 72 plant extracts that directly disrupt the JH receptor complex exhibit JHAN activity.
In a third screening step, we investigated mosquito larvicidal toxicity of selected plant extracts. Ten mosquito third-instar larvae in 3 mL tap water with food mixture were treated with a 500-ppm concentration of each plant extract. Of these 72 extracts, 53 (74%) caused more than 50% larval mortality, suggesting that the larvicidal toxicity of these plant extracts is associated with PJHAN activity (Fig. 1C and Table S1). In contrast, when we tested toxicity of extracts from 110 randomly selected plant species, we found that extracts from only 22 plant species (20%) caused a similar larval mortality (Fig. 1C).
We then purified putative plant juvenile hormone antagonist (PJHAN) compounds from two of the screened plants. The extracts of the two plants were selected based on their availability for collection and the strength of their JHAN activity—specifically, Lindera erythrocarpa fruits and Solidago serotina roots, which have relatively strong and mild activities, respectively (Fig. 1 A and B). Purification resulted in isolation of three diterpene compounds from L. erythrocarpa [LE3B (Kanakugiol), LE3E (methyl linderone), and LE3G (methyl lucidone)] and two diterpenes from S. serotina [SS2A (solidagoic acid A) and SS5A (Kingidiol)] (Fig. 2A) (13–17). Details of the purification process and molecular characterization are shown in Fig. S3 A–O. The molecular structures of these diterpene molecules differed from both the sesquiterpenoid structure of JHs and various terpenoid strucutres of JHAs (Fig. 2A).
These diterpene molecules interfered with pyriproxyfen-mediated Met-CYC binding in the β-galactosidase assay using the Y187 yeast strain in a dose-dependent manner (Fig. 2B). Binding between Met and SRC/FISC/Taiman was observed in A. aegypti (8), T. castaneum (6), and Bombyx mori (18). When we tested whether the plant diterpenes interfered with the pyriproxyfen-mediated binding between A. aegypti Met and FISC, we found that the diterpenes interfered with the JHA-dependent Met-FISC binding with very similar manner to the Met-CYC binding (Fig. 2B). These results show that the diterpenes disrupt both the hormone-mediated heterodimer of Met-CYC and that of Met-FISC. Of the five diterpenes, four also exhibited a significant larvicidal toxicity against third-instar mosquito larvae, with an LD50 of less than 100 ppm at 24 h after treatment (Table 1), yet they were minimally toxic toward human embryonic kidney 293 cells, except for LE3E compound (Fig. S4).
Table 1.
Characteristics of isolated plant diterpenes
| Plant extracts | Compounds | JHAN activity (10 ppm)* | LD50 at 24 h (ppm)† | Effect on development (0.5 μg per insect)* | Impaired gene expression (0.5 μg per insect)* |
| Lindera erythrocarpa fruits | LE3B | 0.89 | 23 | Defect of ovarian follicles‡,§ | Significant reduction |
| LE3E | 0.62 | 47 | ND | NS | |
| LE3G | 0.76 | 31 | ND | NS | |
| Solidago serotina roots | SS2A | 0.39 | >100 | ND | NS |
| SS5A | 0.62 | 85 | Defect of ovarian follicles¶ | Significant reduction | |
| Methoprene acid | 1 | >100 | ND | NS | |
ND, not detected; NS, not significant.
Independent three replication dataset.
Average of independent two replication data.
Control solvent-treated, follicle number per ovary, 102.32 ± 16.14; control follicle size, 102.31 ± 15.76.
LE3B treated, follicle number per ovary, 62.25 ± 10.94 (P = 3.7E-12, t test); control follicle size, 66.41 ± 19.10 (P = 7.0E-49).
SS5A treated, follicle number per ovary, 86.33 ± 6.98 (P = 0.029); control follicle size, 91.27 ± 16.64 (P = 0.054).
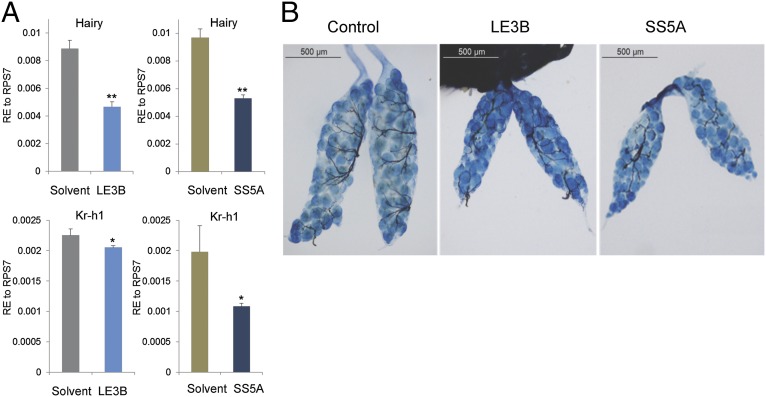
Next, we conducted in vivo tests using the purified diterpene molecules. In the female A. aegypti mosquito, JH removal or Met RNA interference silencing negatively affected the gene expression of two transcriptional regulators: Hairy and Kr-h1 (12). In addition, these treatments resulted in arrest of ovarian development with a significant reduction in the size of ovarian follicles (19). We applied 0.5 μg of either LE3B or SS5A, each purified in 0.2 μL acetone, topically onto the abdomens of newly emerged mosquitoes (12–24 h after eclosion). Acetone was used as a control. Two days after treatment, total RNA was prepared for real-time PCR analysis of kr-h1 and Hairy gene expression. Application of two molecules, LE3B and SS5A, phenocopied the effects of JH and Met depletions. The expression of both Hairy and Kr-h1 were significantly reduced (Fig. 3A). To assess whether these compounds affect development of ovarian follicles, the mosquitoes were dissected 3 d after treatment, after application of either LE3B or SS5A. These treatments resulted in the retardation of ovarian development, with significantly smaller and fewer ovarian follicles than solvent-treated mosquitoes from the control group (Fig. 3B and Table 1). These results indicate that these two diterpenes disrupt JH-dependent regulation. Mosquitoes treated with the other three diterpene molecules exhibited no significant changes in either Hairy or Kr-h1 expression or in the size of ovarian follicles.
Fig. 3.
JHAN activities of LE3B and SS5A in adult female mosquitoes. (A) The impaired expression of JH-activated genes, Hairy and Kr-h1, 48 h after treatment of female mosquitoes with 0.5 μg of these compounds. (B) LE3B and SS5A impaired ovarian development in treated female mosquitoes. (Left) Normal development of ovarian follicles from a female mosquito treated with solvent. (Center and Right) Ovaries from a 4-d-old female mosquito after treatment with 0.5 μg LE3B or SS5A per mosquito.
We also tested the effect of LE3B on mosquito development through longer exposure to second-instar mosquito larvae. We treated them with a sublethal concentration of LE3B (10 ppm), causing 25% (15/20) to 50% (10/20) death overnight (Table S2). In the control group, 90–95% of DMSO-treated larvae survived the night (Table S2). All of the surviving larvae in both the LE3B- or DMSO-treated samples were developed until adult emergence. Four days after emergence, female mosquitoes were dissected to observe ovary development (Fig. S5). We observed that the treatment caused neither morphological disturbance nor lethality through premature or precocious metamorphosis. Instead, we observed early adult emergence (Table S2) and a corresponding defect on ovary development in LE3B-treated mosquitoes (Fig. S5). This observation indicates that the forced early metamorphosis triggered by the plant compound may cause defects in ovary development.
Only LE3B and SS5A among five diterpenes exhibited both in vivo antagonistic activity against JH/JHA on Met target gene expression and ovary development. Although the other three ditepenes and methoprene acid harbored in vitro antagonistic activity against JH/JHA, they did not harbor in vivo antagonistic activity. When we tested whether the plant diterpenes antagonize the JHIII-mediated binding between Met and CYC, we found that LE3B and SS5A interfered more specifically with the natural JH rather than other three diterpenes (Fig. 4). We also observed a similar result when we tested interference by the plant compounds in the JHIII-mediated Met-FISC binding (Fig. 4). This result may contribute to the observed in vivo activity of these two diterpenes. MA did disrupt both JHIII- and pyriproxyfen-mediated binding between Met and its partners, but we could not observe any in vivo antagonistic activity by MA. The molecular details regarding why only LE3B and SS5A had in vivo antagonistic activity against natural JH and the association of JHAN activity of plant extracts and diterpenes with larvicidal toxicity need to be addressed in future studies.
Fig. 4.
Comparison between antagonistic activity against JHIII and one against pyroproxyfen by the plant diterpenes in the hormone-mediated binding of the JH receptor and the partners. The binding initiated by 1 ppm of JHIII or 33 ppb of pyriproxyfen was interfered with 10 ppm of each plant diterpene.
Overall, we found that 53 plant species from 28 families exhibited JHAN activity and larvicidal toxicity against third-instar mosquito larvae (Table S2). Of these samples, four plant families were significantly overrepresented (hypergeometrical distribution, P < 0.01): (i) Araliaceae and (ii) Lauraceae from Archichlamydeae and (iii) Cupressaceae and (iv) Pinaceae from Coniferosida (Table S3). This result suggests that the presence of PJHAN compounds in plants is closely associated with their evolution. Plants deploy secondary metabolites as major constituents of defense against herbivores. It has been suggested that secondary metabolites may improve the producer’s survival fitness by acting at specific receptors in competing organisms (20). All five purified diterpenes belong to secondary metabolites that have no known roles in plant functions. Thus, PJHAN diterpenes likely play a role in plant defense against insect herbivores.
Through using in vitro yeast two-hybrid screening assay and following in vivo results of PJHANs on marker gene expression and ovary development in the mosquito, we demonstrated that they disrupt JH regulation by interfering with the interaction between Met and CYC or FISC. The JH receptors belong to a family of bHLH-PAS transcription factors that is composed of nine phylogenetic clusters (12). Met factors from insects form a unique phylogenetic cluster within the bHLH-PAS protein family. Thus, PJHANs block the endocrine regulation of insect-specific receptors, indicating that they are likely to be environmentally safe. Our results indicate that newly discovered PJHAN compounds could be used as the starting material for development of novel insecticides.
Methods
Yeast Two-Hybrid Binding Test Using Growth Complementation and β-Galactosidase Assay.
The cDNA fragments encoding the full ORF of each Met and CYC from three insects—A. aegypti, C. pipiens, and T. castaneum—and a partial ORF (M1-L689) of T. castaneum SRC were synthesized (Bioneer). Bait plasmids were constructed by introducing each insect Met cDNA into the GAL4 DNA binding domain of the vector pGBKT7 (Clontech). GAL4-AD fusion plasmids with each insect CYC and T. castaneum SRC were constructed as prey plasmids using the pGADT7 vector (Clontech). In addition, a partial ORF (M1-V510) of A. aegypti FISC was cloned into the pGADT7 vector. These prey plasmids were transformed together with the Met bait plasmid into Y2HGold and Y187 yeast cells for the purpose of the yeast two-hybrid binding test using growth complementation and the quantitative β-galactosidase assay, respectively. For growth complementation, binding was tested in synthetic dropout (SD)-Leu/-Trp/-His/-Ade [quadruple dropouts (QDO)] agar medium. The transformed Y187 cells were incubated at 30 °C in SD-Leu/-Trp [double dropouts (DDO)] medium until the OD600 reached 0.3–0.4 and were then harvested and suspended in twice the volume of the media. The cells were further incubated for 2 h, and then 100 µL of the cells in the medium (OD600 = 0.2–0.3) was distributed in 96-well plates. Corresponding concentrations of JH or JHA were added into the growth media, and the cells were incubated for a further 3 h and applied to the β-galactosidase assay using the Yeast β-galactosidase Assay kit (Thermo Scientific). The assay reaction mixtures in the 96-well plates were incubated at 24 °C for 16 h and then centrifuged. The supernatants were subjected to the OD420 measurement.
Screening of Plant Extracts.
The transformed Y187 cells with A. aegypti Met-CYC were grown following the protocol described in the previous section. Then, 100 µL of the grown yeast cells (OD600 = 0.2–0.3) was treated with 33 ppb pyriproxyfen and 100 ppm of each plant extract in 96-well plates. A positive control treated with 33 ppb pyriproxyfen and 10 ppm MA and a negative control treated with 33 ppb pyriproxyfen and control solvent (DMSO) were placed in each tested plate. The cells were incubated for a further 3 h and subjected to the quantitative β-galactosidase assay. The obtained OD420 value for each plant extract-treated sample was converted to an arbitrary unit showing JHAN activity. The OD420 value of the negative control was regarded as the 0 unit and one of the 10 ppm MA-treated samples as the 1 unit. A total of 147 extracts from 101 plant species were screened to determine whether they harbored JHAN activity of greater than 0.3 units. The repeated tests led to extracts of 83 plant species being selected for use in the subsequent screening step
A represents the JHAN activity (if A < 0, then A = 0). The control was treated with 33 ppb pyriproxyfen. The PE group was treated with 33 ppb pyriproxyfen and 100 ppm of each plant extract. The MA10 group was treated with 33 ppb pyriproxyfen and 10 ppm MA.
Growth Inhibition and Anti-Yeast Activity Tests.
The transformed Y2HGold (Clontech) yeast cells with A. aegypti Met-CYC were incubated at 30 °C in DDO (SD-Leu/-Trp) medium until the OD600 reached 0.3–0.4 and then were harvested and suspended in twice the volume of the corresponding media. QDO medium was used for the growth inhibition test, and DDO medium was used for the anti-yeast activity test. The cells were incubated for a further 2 h, and then 200-µL samples were treated with 33 ppb pyriproxyfen and 100 ppm of each screened plant extract in 96-well plates. Each sample was incubated at 30 °C with shaking, and the OD600 of each sample was measured after a 3-h interval.
Extraction, Isolation, and Characterization of Plant Diterpenes.
During October 2013, the fruits of L. erythorocarpa were collected from Jeju Island, Korea, whereas the roots of S. serotina were collected from Hantaek Botanical Garden, Korea. The dried fruits of L. erythorocarpa (58.7 g) and the dried roots of S. serotina (32.7 g) were extracted three times with methanol (1 L) at room temperature to obtain 8.2 and 3.6 g of solid extract, respectively. Each methanol extract was subjected to silica gel column chromatography and was further purified by semipreparative HPLC (Gilson) using a Capcell Pak C18 column (Shiseido Co., Ltd.). LE3B, LE3G, and LE3E were isolated from L. erythorocarpa fruit, whereas SS2A and SS5A were purified from S. serotina roots. The purified active compounds were analyzed using HRESIMS (Waters Q-TOF Premier), 1H NMR, 13C NMR, distortionless enhancement by polarization transfer, heteronuclear multiple quantum coherence, correlation spectroscopy, and heteronuclear multiple bond coherence (Bruker AM500 MHz FT-NMR spectrometer). 1H–NMR, 13C-NMR, high-resolution electrospray ionization mass spectrometry spectrums and ultraperformance liquid chromatography chromatograms of five compounds are shown in Fig. S3. The characterized chemical structures of the compounds are shown in Fig. 2A.
LE3B (Kanakugiol): yellow oil; UV (MeOH) λmax nm 208, 314; 1H NMR (400 MHz, CDCl3) δ 7.91 (1H, d, J = 15.5 Hz, H-α), 7.82 (1H, d, J = 15.5 Hz, H-β), 7.62 (1H, m, H-2 and H-6), 7.40 (1H, m, H-3, H-4, H-5), 4.08 (3H, s, 2′-OCH3), 3.87 (3H, s, 3′-OCH3 and 5′-OCH3), 3.84 (3H, s, 4′-OCH3); 13C NMR (100 MHz, CDCl3) 194.0 (C-β′), 155.2 (C-2'), 153.8 (C-4′), 151.2 (C-6'), 144.4 (C-β), 138.7 (C-5′), 137.5 (C-3′), 135.4 (C-1), 130.7 (C-4), 129.2 (C-3 and C-5), 128.7 (C-2 and C-6), 126.7(C-α), 111.3 (C-1′), 62.4 (C-2′-OCH3), 61.8 (C-5′-OCH3), 61.6 (C-3′-OCH3), 61.3(C-4′-OCH3); HRESIMS m/z [M+H]+ 345.1334, (calculated for C19H21O6, 345.1338).
LE3E (methyl linderone): yellow solid; UV (MeOH) λmax nm 248, 364; 1H NMR (400 MHz, CDCl3) δ 7.92 (1H, d, J = 15.9 Hz, H-α), 7.59 (1H, m, H-2 and H-6), 7.50 (1H, d, J = 15.9 Hz, H-β), 7.36 (1H, m, H-3, H-4, H-5), 4.174 (3H, s, 2′-OCH3), 4.171 (3H, s, 3′-OCH3), 4.08 (3H, s, β′-OCH3); 13C NMR (100 MHz, CDCl3) 187.2 (C-5′), 184.7 (C-2′), 165.4 (C- β′), 149.0 (C-3′), 147.8 (C-4′), 141.2 (C-4), 135.6 (C-1), 130.0 (C-β), 128.9 (C-3 and C-5), 128.3 (C-2 and C-6), 121.2 (C-α), 109.4 (C-1′), 64.3 (C- β′-OCH3) 59.9 (C-2′-OCH3), 59.8 (C-3′-OCH3); HRESIMS m/z [M+H]+ 301.1104, (calculated for C17H17O5, 301.1076).
LE3G (methyl lucidone): yellow solid; UV (MeOH) λmax nm 248, 354; 1H NMR (400 MHz, CDCl3) δ 7.97 (1H, d, J = 15.6 Hz, H-α), 7.60 (1H, d, J = 15.9 Hz, H-β), 7.58 (1H, m, H-2 and H-6), 7.36 (1H, m, H-3, H-4, H-5), 5.92 (1H, s, H-4′), 4.17 (3H, s, β′-OCH3), 3.91 (3H, s, 3′-OCH3); 13C NMR (100 MHz, CDCl3) 191.8 (C-5′), 185.6 (C-2′), 170.2 (C-3′), 169.0 (C- β′), 142.9 (C-β), 135.6 (C-1), 130.5 (C-4), 129.1 (C-3 and C-5), 128.7 (C-2 and C-6), 121.7 (C-α), 111.9 (C-4′), 109.4 (C-1′), 64.8 (C- β′-OCH3), 58.7 (C-3′-OCH3); HRESIMS m/z [M+H]+ 271.0990, (calculated for C16H15O4, 271.0970).
SS2A (solidagoic acid A): white solid; UV (MeOH) λmax nm 217; 1H NMR (400 MHz, CDCl3) δ 7.20 (1H, t, J = 1.6 Hz, H-15), 7.04 (1H, s, H-16), 6.17 (1H, dd, J = 1.6, 0.8 Hz, H-14), 5.52 (H, br s, H-3), 2.56 (1H, m, H-12a), 2.33 (1H, m, H-10), 2.27 (1H, m, H-6a), 2.19 (1H, m, H-12b), 2.07 (2H, m, H-2), 1.74 (2H, m, H-1), 1.59 (1H, m, H-7a, H-8), 1.40 (1H, m, H-6a, H-6b, H-11), 1.50 (3H, s, H-18), 1.29 (1H, m, H-7b), 0.96 (3H, s, H-19) 0.84 (3H, d, J = 6.4 Hz, H-17); 13C NMR (100 MHz, CDCl3) 183.7 (C-20), 142.5 (C-15), 138.7 (C-16), 135.7 (C-4), 125.8 (C-13), 124.2 (C-3), 111.3 (C-14), 51.4 (C-5), 42.0 (C-10), 38.9 (C-9), 37.4 (C-8), 32.4 (C-11), 29.3 (C-6), 28.0 (C-7), 27.1 (C-19), 26.5 (C-2), 19.6 (C-1), 19.1 (C-18), 19.0 (C-12), 16.0 (C-17); HRESIMS m/z [M-H]- 315.1989, (calculated for C20H27O3, 315.1960).
SS5A (Kingidiol): white solid; UV (MeOH) λmax nm 202; 1H NMR (400 MHz, CDCl3) δ 7.32 (1H, t, J = 1.6 Hz, H-15), 7.21 (1H, d, J = 0.4 Hz, H-16), 6.28 (1H, d, J = 0.8 Hz, H-14), 5.89 (1H, t, J = 4.8 Hz, H-3), 4.21 (1H, d, J = 11.2 Hz, H-19a), 3.91 (1H, d, J = 11.2 Hz, H-19b), 3.79 (1H, d, J = 10.6 Hz, H-20a), 3.43 (1H, d, J = 10.6 Hz, H-20b), 2.52 (1H, m, H-12a), 2.31 (1H, m, H-12b), 2.10 (2H, m, H-2), 1.85 (1H, m, H-11a), 1.83 (1H, m, H-1a), 1.81 (1H, m, H-6a), 1.73 (1H, m, H-11b), 1.69 (1H, m, H-1b), 1.62 (1H, m, H-8), 1.49 (1H, m, H-6b, H-7a), 1.35 (1H, m, H-10), 1.32 (1H, m, H-7b), 1.03 (3H, s, H-18), 0.89 (3H, d, J = 6.8 Hz, H-17); 13C NMR (100 MHz, CDCl3) 142.8 (C-15), 141.4 (C-4), 138.7 (C-16), 132.7 (C-3), 126.2 (C-13), 111.3 (C-14), 72.1 (C-20), 64.9 (C-19), 43.3 (C-5), 39.3 (C-10, C-11), 38.9 (C-9), 36.9 (C-8), 27.3 (C-6), 26.9 (C-7), 25.1 (C-2), 24.9 (C-18), 18.8 (C-1), 18.3 (C-12), 15.2 (C-17); HRESIMS m/z [M-H+FA]- 363.2184, (calculated for C20H29O3•CH2O2, 363.2171).
Larval Toxicity Test and LD50 Determination.
Ten mosquito third-instar larvae in 3 mL tap water with food mixture were treated with a 500-ppm concentration of each plant extract. The numbers of dead larvae were counted 24 h after treatment. No dead larva was found in the negative controls treated with the solvent DMSO. All experiments were performed with three replicates, and the average percentage of mortality was counted. For the determination of LD50 of PJHAN compounds, 10 larvae were treated with different concentrations of each compound, with three replicates, and the numbers of dead larvae were counted 24 h after treatment. The average larval mortality data from gradual concentration were subjected to probability unit analysis to calculate LC50.
In Vivo Tests of PJHANs.
Half-microgram samples of the diterpene compounds in 0.2 μL acetone were topically applied onto abdomens of newly emerged mosquitoes. Acetone was used as a control. Two days after treatment, total RNA was prepared for real-time PCR analysis of kr-h1 and Hairy gene expression. To assess the development of ovarian follicles, the mosquitoes were dissected 3 d after treatment. To test the prolonged effect of LE3B, 20 mosquito second-instar larvae in 10 mL tap water with food mixture were treated with a 10-ppm concentration of LE3B. The numbers of dead larvae were counted 24 h after treatment, and the survived larvae were maintained at 26 °C until adult emergence. The density of larvae was adjusted by adding more tap water with food mixture to control samples. Then, the larvae were left until adult emergence and then the ovaries of the emerged female mosquitoes were dissected 4 d after emergence.
RNA Preparation and Real-Time RT-PCR.
Total RNA was prepared using TRIzol (GIBCO/BRL). Real-time RT-PCR experiments were performed following a previously described method (8).
Maintenance of Mosquitoes and Vertebrate Approval.
The mosquito A. aegypti UGAL/Rockefeller strain was raised as described previously (21), and all animal experiments were performed with the approval of the Institutional Animal Use and Care Committee of the Korea Research Institute of Bioscience and Biotechnology.
Supplementary Material
Acknowledgments
We thank Yunhee Shin for help in editing the manuscript. This work was supported by grants from Seoul National University (to S.W.S.); Next-Generation Biogreen 21 Program Grant PJ009031 (to Y.H.J.); KRIBB Research Initiative Program Grant KGM2131413 (to H.-W.O.), and National Institutes of Health Grant R01 AI036959 (to A.S.R.). S.-H.L., Y.F., and S.-B.A. were supported by the second stage of the Brain Korea 21 Project.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1424386112/-/DCSupplemental.
References
- 1.Hill CA, Kafatos FC, Stansfield SK, Collins FH. Arthropod-borne diseases: Vector control in the genomics era. Nat Rev Microbiol. 2005;3(3):262–268. doi: 10.1038/nrmicro1101. [DOI] [PubMed] [Google Scholar]
- 2.Boyer S, Zhang H, Lempérière G. A review of control methods and resistance mechanisms in stored-product insects. Bull Entomol Res. 2012;102(2):213–229. doi: 10.1017/S0007485311000654. [DOI] [PubMed] [Google Scholar]
- 3.Pener M, Dhadialla TS. An overview of insect growth disruptors: Applied aspects.eds Dhadialla TS. Advances in Insect Physiology. 2012:1–162. (Elsevier, Oxford), Vol 43, pp . [Google Scholar]
- 4.Sláma K. Insect juvenile hormone analogues. Annu Rev Biochem. 1971;40:1079–1102. doi: 10.1146/annurev.bi.40.070171.005243. [DOI] [PubMed] [Google Scholar]
- 5.Charles JP, et al. Ligand-binding properties of a juvenile hormone receptor, Methoprene-tolerant. Proc Natl Acad Sci USA. 2011;108(52):21128–21133. doi: 10.1073/pnas.1116123109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jindra M, Palli SR, Riddiford LM. The juvenile hormone signaling pathway in insect development. Annu Rev Entomol. 2013;58:181–204. doi: 10.1146/annurev-ento-120811-153700. [DOI] [PubMed] [Google Scholar]
- 7.Kewley RJ, Whitelaw ML, Chapman-Smith A. The mammalian basic helix-loop-helix/PAS family of transcriptional regulators. Int J Biochem Cell Biol. 2004;36(2):189–204. doi: 10.1016/s1357-2725(03)00211-5. [DOI] [PubMed] [Google Scholar]
- 8.Li M, Mead EA, Zhu J. Heterodimer of two bHLH-PAS proteins mediates juvenile hormone-induced gene expression. Proc Natl Acad Sci USA. 2011;108(2):638–643. doi: 10.1073/pnas.1013914108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shin SW, Zou Z, Saha TT, Raikhel AS. bHLH-PAS heterodimer of methoprene-tolerant and Cycle mediates circadian expression of juvenile hormone-induced mosquito genes. Proc Natl Acad Sci USA. 2012;109(41):16576–16581. doi: 10.1073/pnas.1214209109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sláma K, Williams CM. ‘Paper factor’ as an inhibitor of the embryonic development of the European bug, Pyrrhocoris apterus. Nature. 1966;210(5033):329–330. doi: 10.1038/210329a0. [DOI] [PubMed] [Google Scholar]
- 11.Bowers WS, Fales HM, Thompson MJ, Uebel EC. Juvenile hormone: Identification of an active compound from balsam fir. Science. 1966;154(3752):1020–1021. doi: 10.1126/science.154.3752.1020. [DOI] [PubMed] [Google Scholar]
- 12.Bowers WS. 1991. Insect hormones and antihormones in plants. Herbivores: Their Interactions with Secondary Plant Metabolites, Vol. I, eds. Rosenthal GA, Berenbaum MR (Academic Press, New York)
- 13.Lee SM, Baek SH, Lee CH, Lee HB, Kho YH. Cytotoxicity of lignans from Lindera erytherocarpa Makino. Nat Prod Sci. 2002;8(3):100–102. [Google Scholar]
- 14.Ng S. 13C NMR study on linderones and lucidones. Magn Reson Chem. 1990;28(4):337–342. [Google Scholar]
- 15.Anthonsen T, et al. Constituents of Solidago species. Part IV. Solidagoic acids A and B, diterpenoids from Solidago gigantea var. serotina. Can J Chem. 1973;51(9):1332–1345. [Google Scholar]
- 16.Bohlmann F, Zdero C, King RM, Robinson H. Kingidiol, a kolavane derivative from Baccharis kingii. Phytochmiestry. 1984;23(7):1511–1512. [Google Scholar]
- 17.Sosa ME, Tonn CE, Giordano OS. Insect antifeedant activity of clerodane diterpenoids. J Nat Prod. 1994;57(9):1262–1265. doi: 10.1021/np50111a012. [DOI] [PubMed] [Google Scholar]
- 18.Kayukawa T, et al. Transcriptional regulation of juvenile hormone-mediated induction of Krüppel homolog 1, a repressor of insect metamorphosis. Proc Natl Acad Sci USA. 2012;109(29):11729–11734. doi: 10.1073/pnas.1204951109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zou Z, et al. Juvenile hormone and its receptor, methoprene-tolerant, control the dynamics of mosquito gene expression. Proc Natl Acad Sci USA. 2013;110(24):E2173–E2181. doi: 10.1073/pnas.1305293110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams DH, Stone MJ, Hauck PR, Rahman SK. Why are secondary metabolites (natural products) biosynthesized? J Nat Prod. 1989;52(6):1189–1208. doi: 10.1021/np50066a001. [DOI] [PubMed] [Google Scholar]
- 21.Roy SG, Hansen IA, Raikhel AS. Effect of insulin and 20-hydroxyecdysone in the fat body of the yellow fever mosquito, Aedes aegypti. Insect Biochem Mol Biol. 2007;37(12):1317–1326. doi: 10.1016/j.ibmb.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.