Abstract
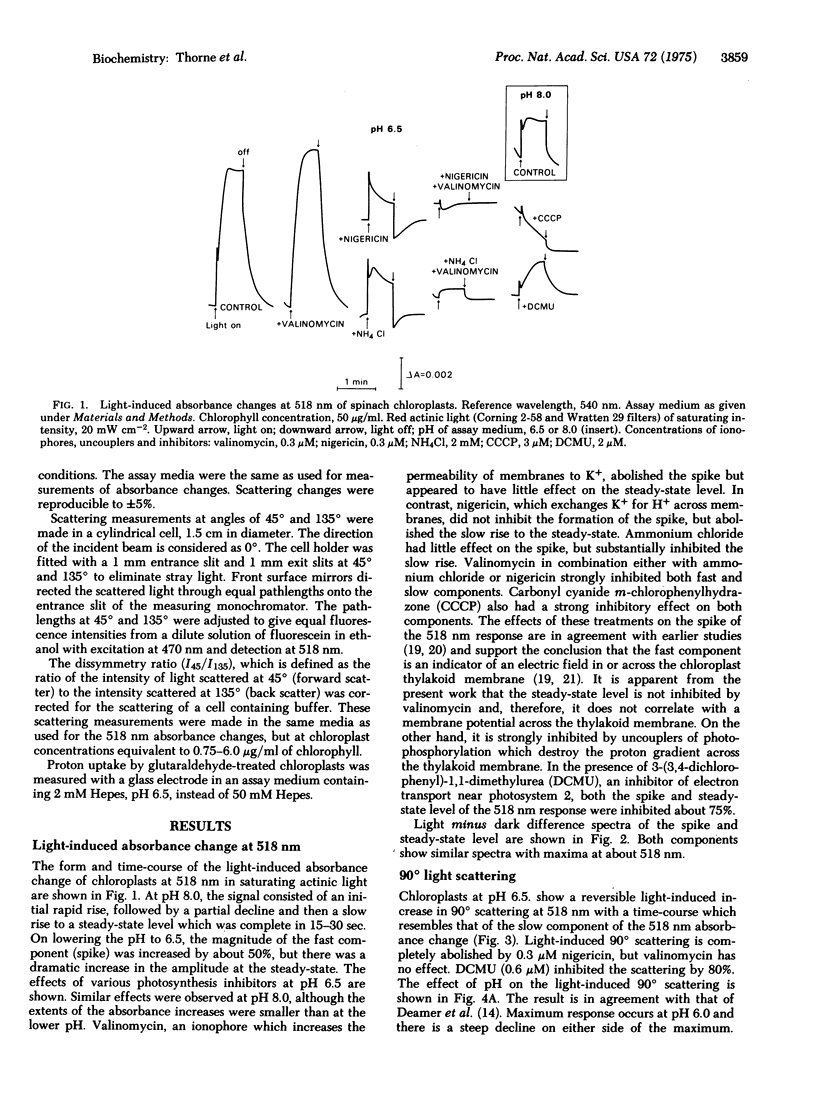
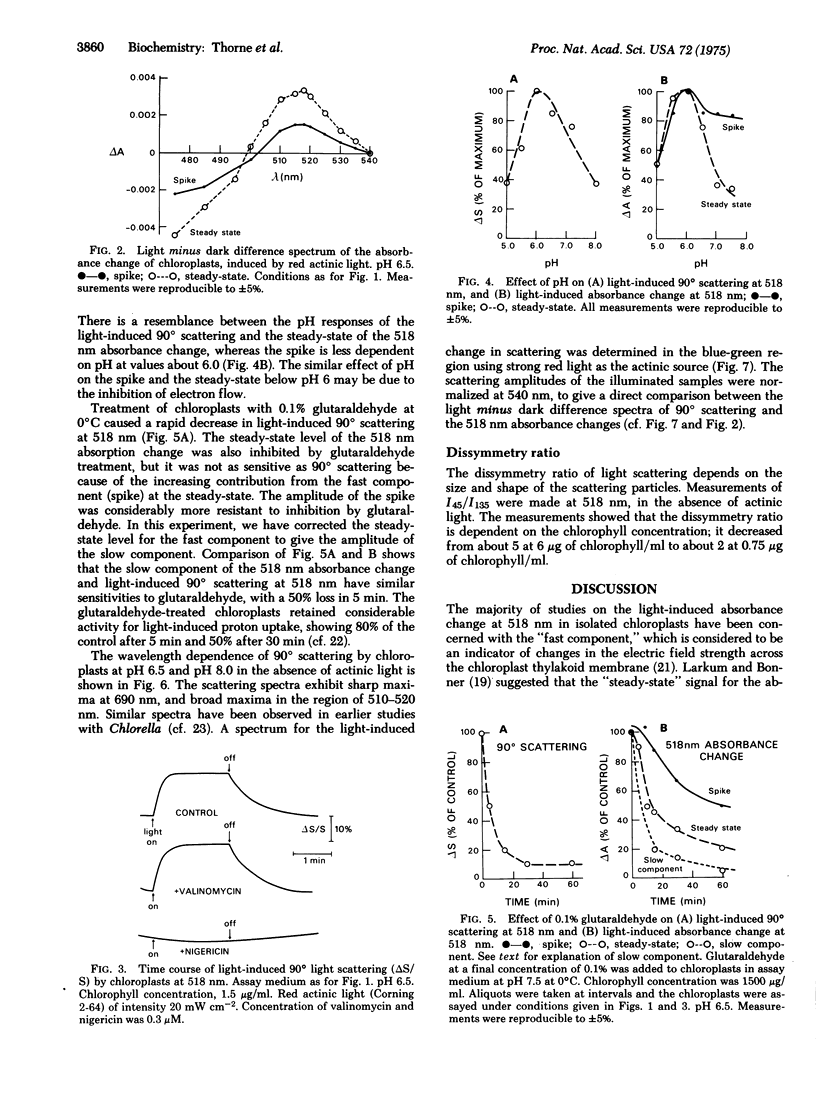
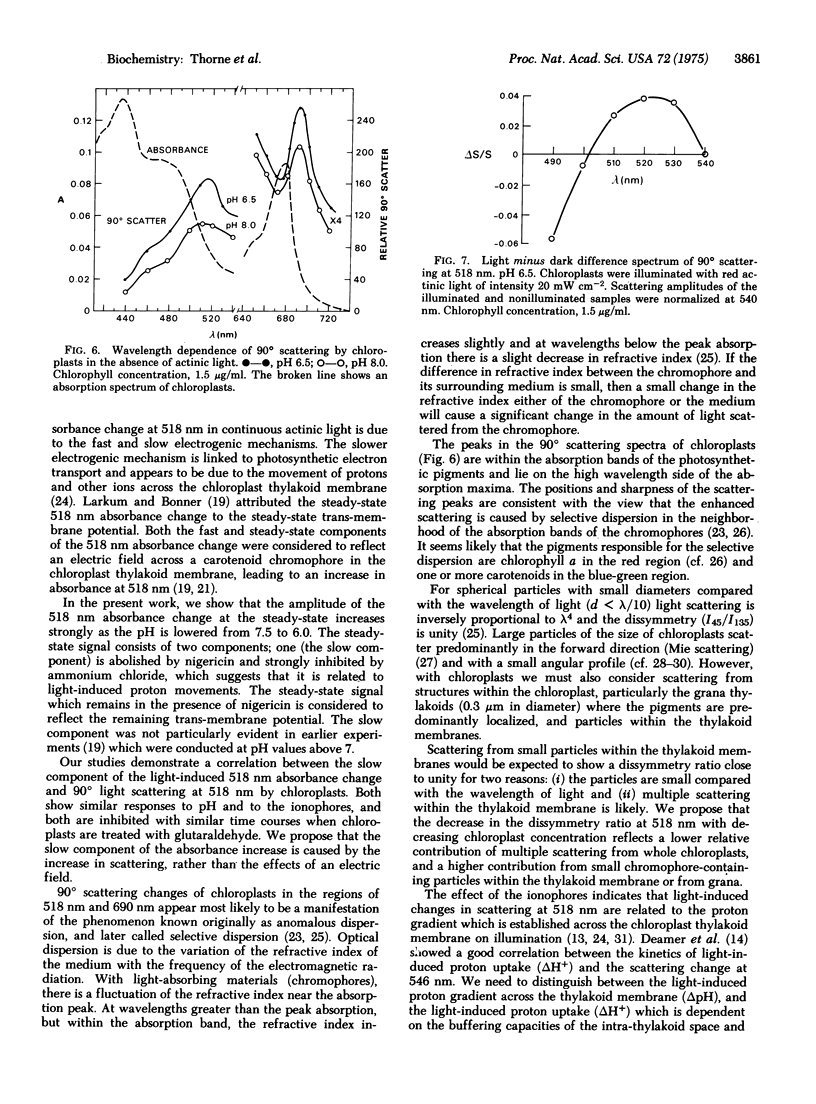
The light-induced absorbance change at 518 nm of isolated chloroplasts consists of a rapid phase, and a slow phase which is complete in about 20 sec. The slow component of the 518 nm absorbance change correlates with the light-induced change in 90 degrees light scattering at 518 nm. Both show a similar time course, similar pH dependence with a maximum at pH 6.0, and similar sensitivity to inhibitors and to treatment of the chloroplasts with a low concentration of glutaraldehyde. Their light minus dark difference spectra are similar with maxima at about 520 nm. It is concluded that they are manifestations of the same phenomenon, and the slow absorbance increase at 518 nm is due to enhanced scattering. It is proposed that the light-induced changes in scattering at 518 nm reflect alterations in selective dispersion, due to proton uptake and conformational changes in the chloroplast thylakoid membrane.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boardman N. K., Thorne S. W. Studies on a barley mutant lacking chlorophyll b. II. Fluorescence properties of isolated chloroplasts. Biochim Biophys Acta. 1968 Feb 12;153(2):448–458. doi: 10.1016/0005-2728(68)90086-8. [DOI] [PubMed] [Google Scholar]
- Bryant F. D., Seiber B. A., Latimer P. Absolute optical cross sections of cells and chloroplasts. Arch Biochem Biophys. 1969 Dec;135(1):97–108. doi: 10.1016/0003-9861(69)90520-7. [DOI] [PubMed] [Google Scholar]
- DILLEY R. A., VERNON L. P. CHANGES IN LIGHT-ABSORPTION AND LIGHT-SCATTERING PROPERTIES OF SPINACH CHLOROPLASTS UPON ILLUMINATION: RELATIONSHIP TO PHOTOPHOSPHORYLATION. Biochemistry. 1964 Jun;3:817–824. doi: 10.1021/bi00894a016. [DOI] [PubMed] [Google Scholar]
- Gregory R. P., Raps S. The differential scattering of circularly polarized light by chloroplasts and evaluation of their true circular dichroism. Biochem J. 1974 Aug;142(2):193–201. doi: 10.1042/bj1420193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber U. Conformational changes of chloroplasts induced by illumination of leaves in vivo. Biochim Biophys Acta. 1969 Jun 24;180(2):302–319. doi: 10.1016/0005-2728(69)90116-9. [DOI] [PubMed] [Google Scholar]
- Heber U. Stoichiometry of reduction and phosphorylation during illumination of intact chloroplasts. Biochim Biophys Acta. 1973 Apr 27;305(1):140–152. doi: 10.1016/0005-2728(73)90239-9. [DOI] [PubMed] [Google Scholar]
- KOCH A. L. Some calculations on the turbidity of mitochondria and bacteria. Biochim Biophys Acta. 1961 Aug 19;51:429–441. doi: 10.1016/0006-3002(61)90599-6. [DOI] [PubMed] [Google Scholar]
- KUSHIDA H., ITOH M., IZAWA S., SHIBATA K. DEFORMATIONS OF CHLOROPLASTS ON ILLUMINATION IN INTACT SPINACH LEAVES. Biochim Biophys Acta. 1964 Jan 27;79:201–203. doi: 10.1016/0926-6577(64)90051-8. [DOI] [PubMed] [Google Scholar]
- LATIMER P., RABINOWITCH E. Selective scattering of light by pigments in vivo. Arch Biochem Biophys. 1959 Oct;84:428–441. doi: 10.1016/0003-9861(59)90605-8. [DOI] [PubMed] [Google Scholar]
- Larkum A. W., Boardman N. K. The effect of nigericin and valinomycin on CO2 fixation electron transport and P518 in intact spinach chloroplasts. FEBS Lett. 1974 Mar 15;40(1):229–232. doi: 10.1016/0014-5793(74)80934-8. [DOI] [PubMed] [Google Scholar]
- Larkum A. W., Bonner W. D. Light-induced absorbance changes of P518 in intact chloroplasts. Biochim Biophys Acta. 1972 Feb 28;256(2):396–408. doi: 10.1016/0005-2728(72)90070-9. [DOI] [PubMed] [Google Scholar]
- Latimer P. Influence of Selective Light Scattering on Measurements of Absorption Spectra of Chlorella. Plant Physiol. 1959 May;34(3):193–199. doi: 10.1104/pp.34.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Nobel P. S. Light-Induced Chloroplast Shrinkage in vivo Detectable After Rapid Isolation of Chloroplasts From Pisum sativum. Plant Physiol. 1968 May;43(5):781–787. doi: 10.1104/pp.43.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PACKER L. Light scattering changes correlated with photosynthetic phosphorylation in chloroplast fragments. Biochem Biophys Res Commun. 1962 Oct 31;9:355–360. doi: 10.1016/0006-291x(62)90054-2. [DOI] [PubMed] [Google Scholar]
- Packer L., Allen J. M., Starks M. Light-induced ion tranpsort in glutaraldehyde-fixed chloroplasts: studies with nigericin. Arch Biochem Biophys. 1968 Oct;128(1):142–152. doi: 10.1016/0003-9861(68)90017-9. [DOI] [PubMed] [Google Scholar]
- Packer L., Siegenthaler P. A. Control of chloroplast structure by light. Int Rev Cytol. 1966;20:97–124. doi: 10.1016/s0074-7696(08)60798-6. [DOI] [PubMed] [Google Scholar]
- Reeves S. G., Hall D. O. The stoichiometry (ATP-2e- ratio) of non-cyclic photophosphorylation in isolated spinach chloroplasts. Biochim Biophys Acta. 1973 Jul 26;314(1):66–78. doi: 10.1016/0005-2728(73)90064-9. [DOI] [PubMed] [Google Scholar]
- Rottenberg H., Grunwald T., Avron M. Determination of pH in chloroplasts. I. Distribution of ( 14 C) methylamine. Eur J Biochem. 1972 Jan 31;25(1):54–63. doi: 10.1111/j.1432-1033.1972.tb01666.x. [DOI] [PubMed] [Google Scholar]
- Verkhoturov V. N., Tulbu G. V. Indutsirovannoe svetom izmenenie rasseivaiushchikh svoistv khloroplastov list'ev gorokha. Biofizika. 1973 Nov-Dec;18(6):1052–1057. [PubMed] [Google Scholar]
- West J., Packer L. The effect of glutaraldehyde on light-induced H+ changes, electron transport, and phosphorylation in pea chloroplasts. J Bioenerg. 1970 Oct;1(4):405–412. doi: 10.1007/BF01654577. [DOI] [PubMed] [Google Scholar]
- West K. R., Wiskich J. T. Photosynthetic control by isolated pea chloroplasts. Biochem J. 1968 Oct;109(4):527–532. doi: 10.1042/bj1090527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt H. T. Coupling of quanta, electrons, fields, ions and phosphrylation in the functional membrane of photosynthesis. Results by pulse spectroscopic methods. Q Rev Biophys. 1971 Nov;4(4):365–477. doi: 10.1017/s0033583500000834. [DOI] [PubMed] [Google Scholar]


