Abstract
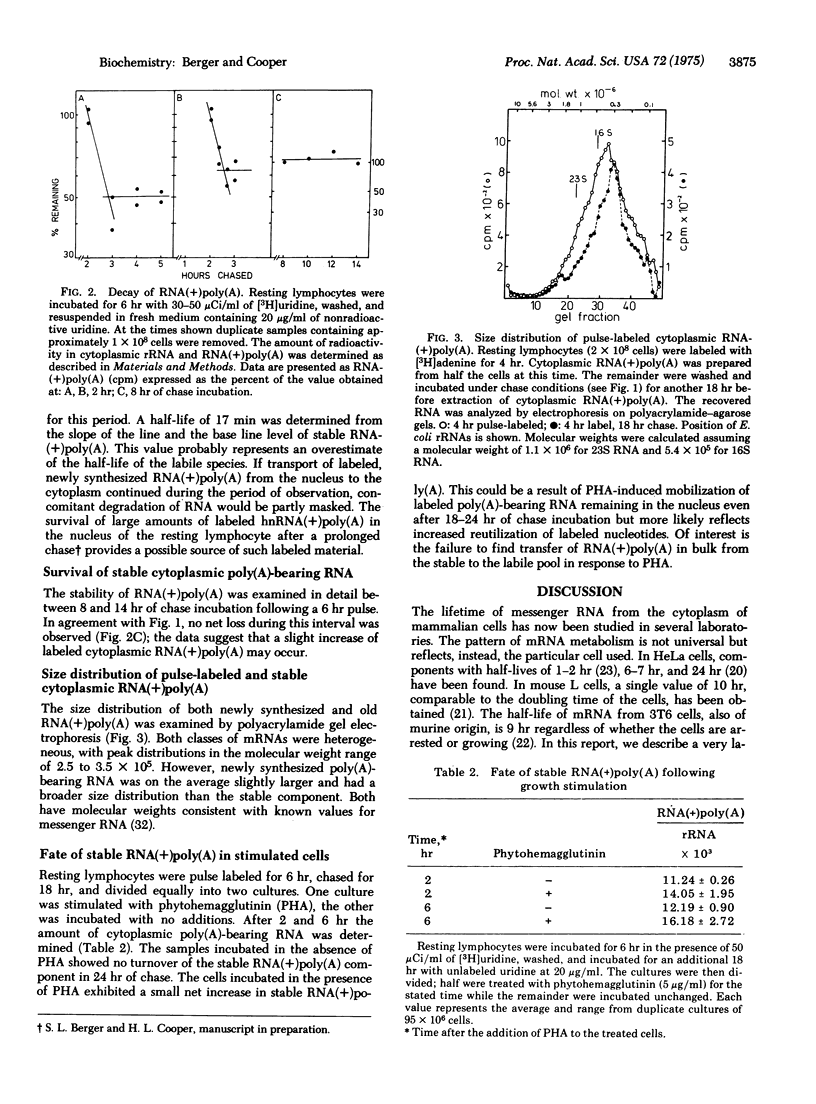
The kinetics of degradation of newly synthesized cytoplasmic poly(A)-bearing RNA have been examined in resting human lymphocytes. Two classes were identified, a very labile component with a half-life of less than 17 min and a stable component which remains apparently undiminished during 24 hr of observation. Both classes have molecular weights between 2.5 and 3.5 x 10(5) but the stable material has a narrower size distribution and a slightly lower average molecular weight than the short-lived component. The fate of stable RNA synthesized in the resting cell was also examined after growth stimulation with phytohemagglutinin after 2 and 6 hr of treatment. No transfer of stable material into the labile pool could be discerned; the amount of stable material remained constant. The existence of two species of mRNAs with different lifetimes in animal cells provides a potential means for regulation of protein synthesis by controlling the supply of specific messages. Furthermore, such a short-lived mRNA species may explain the observed disparity between the amount of poly(A)-bearing heterogeneous RNA produced in the nucleus and the amount of mature message found in the cytoplasm.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham G., Rhodes D. P., Banerjee A. K. Novel initiation of RNA synthesis in vitro by vesicular stomatitis virus. Nature. 1975 May 1;255(5503):37–40. doi: 10.1038/255037a0. [DOI] [PubMed] [Google Scholar]
- Adams J. M., Cory S. Modified nucleosides and bizarre 5'-termini in mouse myeloma mRNA. Nature. 1975 May 1;255(5503):28–33. doi: 10.1038/255028a0. [DOI] [PubMed] [Google Scholar]
- Both G. W., Banerjee A. K., Shatkin A. J. Methylation-dependent translation of viral messenger RNAs in vitro. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1189–1193. doi: 10.1073/pnas.72.3.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone R. E., Marchalonis J. J. Surface proteins of thymus-derived lymphocytes and bone-marrow-derived lymphocytes. Selective isolation of immunoglobulin and the theta-antigen by non-ionic detergents. Biochem J. 1974 Jun;140(3):345–354. doi: 10.1042/bj1400345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell J. E., Jelinek W. R., Molloy G. R. Biogenesis of mRNA: genetic regulation in mammalian cells. Science. 1973 Sep 28;181(4106):1215–1221. doi: 10.1126/science.181.4106.1215. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Philipson L., Wall R., Adesnik M. Polyadenylic acid sequences: role in conversion of nuclear RNA into messenger RNA. Science. 1971 Oct 29;174(4008):507–510. doi: 10.1126/science.174.4008.507. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Wall R., Tushinski R. J. An adenylic acid-rich sequence in messenger RNA of HeLa cells and its possible relationship to reiterated sites in DNA. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1321–1325. doi: 10.1073/pnas.68.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds M., Vaughan M. H., Jr, Nakazato H. Polyadenylic acid sequences in the heterogeneous nuclear RNA and rapidly-labeled polyribosomal RNA of HeLa cells: possible evidence for a precursor relationship. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1336–1340. doi: 10.1073/pnas.68.6.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi Y., Morgan M., Shatkin A. J., Jelinek W., Salditt-Georgieff M., Darnell J. E. Methylated, blocked 5 termini in HeLa cell mRNA. Proc Natl Acad Sci U S A. 1975 May;72(5):1904–1908. doi: 10.1073/pnas.72.5.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilham P. T. The synthesis of celluloses containing covalently bound nucleotides, polynucleotides, and nucleic acids. Biochemistry. 1968 Aug;7(8):2809–2813. doi: 10.1021/bi00848a016. [DOI] [PubMed] [Google Scholar]
- Greenberg J. R. High stability of messenger RNA in growing cultured cells. Nature. 1972 Nov 10;240(5376):102–104. doi: 10.1038/240102a0. [DOI] [PubMed] [Google Scholar]
- Imaizumi T., Diggelmann H., Scherrer K. Demonstration of globin messenger sequences in giant nuclear precursors of messenger RNA of avian erythroblasts. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1122–1126. doi: 10.1073/pnas.70.4.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek W., Adesnik M., Salditt M., Sheiness D., Wall R., Molloy G., Philipson L., Darnell J. E. Further evidence on the nuclear origin and transfer to the cytoplasm of polyadenylic acid sequences in mammalian cell RNA. J Mol Biol. 1973 Apr 15;75(3):515–532. doi: 10.1016/0022-2836(73)90458-0. [DOI] [PubMed] [Google Scholar]
- Jondal M., Wigzell H., Aiuti F. Human lymphocyte subpopulations: classification according to surface markers and-or functional characteristics. Transplant Rev. 1973;16:163–195. doi: 10.1111/j.1600-065x.1973.tb00120.x. [DOI] [PubMed] [Google Scholar]
- Lee S. Y., Mendecki J., Brawerman G. A polynucleotide segment rich in adenylic acid in the rapidly-labeled polyribosomal RNA component of mouse sarcoma 180 ascites cells. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1331–1335. doi: 10.1073/pnas.68.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin B. Units of transcription and translation: sequence components of heterogeneous nuclear RNA and messenger RNA. Cell. 1975 Feb;4(2):77–93. doi: 10.1016/0092-8674(75)90113-0. [DOI] [PubMed] [Google Scholar]
- Lewin B. Units of transcription and translation: the relationship between heterogeneous nuclear RNA and messenger RNA. Cell. 1975 Jan;4(1):11–20. doi: 10.1016/0092-8674(75)90128-2. [DOI] [PubMed] [Google Scholar]
- McKnight G. S., Schimke R. T. Ovalbumin messenger RNA: evidence that the initial product of transcription is the same size as polysomal ovalbumin messenger. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4327–4331. doi: 10.1073/pnas.71.11.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendecki J., Lee S. Y., Brawerman G. Characteristics of the polyadenylic acid segment associated with messenger ribonucleic acid in mouse sarcoma 180 ascites cells. Biochemistry. 1972 Feb 29;11(5):792–798. doi: 10.1021/bi00755a018. [DOI] [PubMed] [Google Scholar]
- Molloy G. R., Darnell J. E. Characterization of the poly(adenylic acid) regions and the adjacent nucleotides in heterogeneous nuclear ribonucleic acid and messenger ribonucleic acid from HeLa cells. Biochemistry. 1973 Jun 5;12(12):2324–2330. doi: 10.1021/bi00736a022. [DOI] [PubMed] [Google Scholar]
- Muthukrishnan S., Both G. W., Furuichi Y., Shatkin A. J. 5'-Terminal 7-methylguanosine in eukaryotic mRNA is required for translation. Nature. 1975 May 1;255(5503):33–37. doi: 10.1038/255033a0. [DOI] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- Penman S., Rosbash M., Penman M. Messenger and heterogeneous nuclear RNA in HeLa cells: differential inhibition by cordycepin. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1878–1885. doi: 10.1073/pnas.67.4.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E., LaTorre J. Synthesis and turnover of nuclear and cytoplasmic polyadenylic acid in mouse L cells. J Mol Biol. 1974 Jan 25;82(3):315–331. doi: 10.1016/0022-2836(74)90593-2. [DOI] [PubMed] [Google Scholar]
- Puckett L., Chambers S., Darnell J. E. Short-lived messenger RNA in HeLa cells and its impace on the kinetics of accumulation of cytoplasmic polyadenylate. Proc Natl Acad Sci U S A. 1975 Jan;72(1):389–393. doi: 10.1073/pnas.72.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottman F., Shatkin A. J., Perry R. P. Sequences containing methylated nucleotides at the 5' termini of messenger RNAs: possible implications for processing. Cell. 1974 Nov;3(3):197–199. doi: 10.1016/0092-8674(74)90131-7. [DOI] [PubMed] [Google Scholar]
- Ruiz-Carrilo A., Beato M., Schutz G., Feigelson P., Allfrey V. G. Cell-free translation of the globin message within polydisperse high-molecular-weight ribonucleic acid of avian erythrocytes. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3641–3645. doi: 10.1073/pnas.70.12.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheiness D., Darnell J. E. Polyadenylic acid segment in mRNA becomes shorter with age. Nat New Biol. 1973 Feb 28;241(113):265–268. doi: 10.1038/newbio241265a0. [DOI] [PubMed] [Google Scholar]
- Sheiness D., Puckett L., Darnell J. E. Possible relationship of poly(A) shortening to mRNA turnover. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1077–1081. doi: 10.1073/pnas.72.3.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer R. H., Penman S. Messenger RNA in HeLa cells: kinetics of formation and decay. J Mol Biol. 1973 Aug 5;78(2):321–334. doi: 10.1016/0022-2836(73)90119-8. [DOI] [PubMed] [Google Scholar]
- Spradling A., Hui H., Penman S. Two very different components of messenger RNA in an insect cell line. Cell. 1975 Feb;4(2):131–137. doi: 10.1016/0092-8674(75)90119-1. [DOI] [PubMed] [Google Scholar]
- Stevens R. H., Williamson A. R. Isolation of nuclear pre-mRNA which codes for immunoglobulin heavy chain. Nat New Biol. 1973 Sep 26;245(143):101–104. doi: 10.1038/newbio245101a0. [DOI] [PubMed] [Google Scholar]
- Stevens R. H., Williamson A. R. Specific IgG mRNA molecules from myeloma cells in heterogeneous nuclear and cytoplasmic RNA containing poly-A. Nature. 1972 Sep 15;239(5368):143–146. doi: 10.1038/239143a0. [DOI] [PubMed] [Google Scholar]
- Wall R., Weber J., Gage Z., Darnell J. E. Production of viral mRNA in adenovirus- transformed cells by the post- transcriptional processing of heterogeneous nuclear RNA containing viral and cell sequences. J Virol. 1973 Jun;11(6):953–960. doi: 10.1128/jvi.11.6.953-960.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C. M., Gershowitz A., Moss B. Methylated nucleotides block 5' terminus of HeLa cell messenger RNA. Cell. 1975 Apr;4(4):379–386. doi: 10.1016/0092-8674(75)90158-0. [DOI] [PubMed] [Google Scholar]
- Williamson R., Drewienkiewicz C. E., Paul J. Globin messenger sequences in high molecular weight RNA from embryonic mouse liver. Nat New Biol. 1973 Jan 17;241(107):66–68. doi: 10.1038/newbio241066a0. [DOI] [PubMed] [Google Scholar]


