Abstract
Introduction
Cotrimoxazole is widely prescribed to treat a range of infections and for HIV-infected individuals it is administered as prophylaxis to protect against opportunistic infections. Some reports suggest that fetuses exposed to cotrimoxazole during early pregnancy may have an increased risk of congenital anomalies. We carried out this systematic review in order to update the evidence of cotrimoxazole safety in pregnancy.
Methods
Three databases and one conference abstract site were searched in duplicate up to 31 October, 2013 for studies reporting adverse maternal and infant outcomes among women receiving cotrimoxazole during pregnancy. This search was updated in MEDLINE via PUBMED to 28 April 2014. Studies were included irrespective of HIV-infection status or the presence of other co-infections. Our primary outcome was birth defects of any kind. Secondary outcomes included spontaneous abortions, terminations of pregnancy, stillbirths, preterm deliveries, and drug-associated toxicity.
Results
24 studies were included for review. There were 232 infants with congenital anomalies among 4196 women receiving cotrimoxazole during pregnancy, giving an overall pooled prevalence of 3.5% (95% CI 1.8–5.1%; τ2 0.03). Three studies reported 31 infants with neural tube defects, giving a crude prevalence of 0.7% (95%CI 0.5–1.0%) with most data (29 neural tube defects) coming from a single study. The majority of adverse drug reactions were mild. The quality of the evidence was very low.
Conclusions
The findings of this review support continued recommendations for cotrimoxazole as a priority intervention for HIV-infected pregnant women. It is critical to improve data collection on maternal and infant outcomes.
Keywords: birth defects, congenital anomalies, cotrimoxazole, HIV/AIDS, pregnancy
Introduction
Cotrimoxazole (trimethoprim–sulfamethoxazole) is a safe, effective, low-cost combination antibiotic that is widely prescribed to treat a range of bacterial, parasitic, and fungal infections. For HIV-infected individuals, cotrimoxazole administered as prophylaxis provides protection against the opportunistic infection pathogens Pneumocystis jirovecii and Toxoplasma gondii. It has also been shown to be protective against malaria, bacterial pneumonia and diarrheal disease in resource-limited countries, resulting in a reduced risk of death in clinical trials in these settings.1,2 For HIV-infected pregnant women, the use of prophylactic cotrimoxazole is associated with a reduction in preterm delivery and neonatal mortality in their HIV-exposed infants.3 Since 2006, the World Health Organization (WHO) has recommended that cotrimoxazole prophylaxis should be provided to all HIV-infected individuals with a CD4 cell count <350 per mm3, particularly in resource-limited settings where bacterial infections and malaria are prevalent.4
Cotrimoxazole provides sequential and synergistic inhibition of bacterial folate metabolism through its action on dihydropteroate synthesase and dihydrofolate reductase (DHFR) enzymes, inhibiting the biosynthesis of nucleic acids. Although more selective for the bacterial than the human DHFR isoenzyme, the drug can nevertheless interfere with human folate metabolism.5 Pregnancy is associated with rapid cell division in the unborn child, and folate is essential for fetal development because of its critical role in DNA synthesis.6 Folate deficiency in early pregnancy is associated with adverse pregnancy outcomes, including an increased risk of neural tube defects and other congenital defects.7 Both drugs, trimethoprim and sulfamethoxazole, cross the placental barrier, reaching peak fetal levels within 3 hours of administration; fetal levels of sulfamethoxazole average 70–90% of maternal levels while those of trimethoprim are comparable to maternal levels.8,9 Pregnancy exposure studies carried out in rats and rabbits10 and small, retrospective studies in humans have reported some evidence of congenital anomalies with first trimester cotrimoxazole exposure.11 Cotrimoxazole is listed as a Class D drug by the Food and Drug Administration12,13 meaning that there is positive evidence of human fetal risk based on adverse reaction data from investigational or marketing experience or from studies in humans, but the potential benefits of the drug may warrant its use in pregnancy despite the potential risks.
In the United States, guidelines for the management of HIV-infected adults and adolescents acknowledge a possible association between first-trimester exposure to trimethoprim and an increased risk of congenital anomalies; cotrimoxazole use in the first-trimester of pregnancy is still recommended for the treatment of Pneumocystis pneumonia because of its considerable benefit, while for prophylaxis the guidelines state that health care providers may consider using alternative regimens.14 WHO’s 2006 guidelines recommend that women who fulfill the criteria for cotrimoxazole prophylaxis should continue cotrimoxazole throughout their pregnancy, since the risk of life-threatening infections outweighs the potential risk of congenital abnormalities.4
We carried out this systematic review in order to update the evidence of cotrimoxazole safety in pregnancy to inform a revision of WHO’s guidelines for cotrimoxazole prophylaxis in pregnant women infected with HIV.
Methods
Search strategy and study selection
Using a pre-defined protocol incorporating a compound search strategy (Supplementary Appendix 1), we searched EMBASE, MEDLINE via PubMed and The Cochrane Library up to 31 October, 2013 for studies reporting adverse maternal and infant outcomes among women exposed to cotrimoxazole during pregnancy. The search was updated in MEDLINE via PubMed up to 28 April 2014. We also reviewed online abstracts of all conferences of the International AIDS Society using single terms for cotrimoxazole (up to Kuala Lumpur, June 2013) and hand searched bibliographies of previously published systematic and non-systematic reviews and other relevant articles. No language or geographical restrictions were applied.
Two reviewers (N.F, Z.S.), working independently, scanned all titles for eligibility according to predefined inclusion criteria. Once all potentially relevant full-text articles and abstracts were identified, we consulted clinical experts (L.M., E.A, J. J.) to achieve consensus regarding eligibility criteria. Studies were included irrespective of HIV-infection status or the presence of other co-infections. Where infections were associated with outcomes under assessment (e.g. brucellosis and stillbirth), however, we did not pool data due to confounding by indication. We made no distinction regarding whether cotrimoxazole was provided for prophylaxis or treatment. Studies assessing sulfonamide drugs alone were excluded.
Data extraction
Data extraction was conducted independently and in duplicate using a pre-piloted data extraction form (Z.S., N.F.) and subsequently verified by two other reviewers (J.J., L.M.). Information was extracted on study size, setting, and population; co-infection status; period and duration of exposure; and birth outcomes. Our primary outcome was birth defects of any kind. Secondary outcomes included spontaneous abortions, terminations of pregnancy, stillbirths, preterm deliveries, and drug-associated toxicity.
Assessment of methodological quality
Risk of bias was assessed according to six criteria: direct ascertainment of cotrimoxazole use, adjustment for confounders, prospective study design, outcomes reported by trimester, outcomes reported by folate supplement use, and potential for confounding by indication. This risk of bias assessment was used to inform the overall assessment of the quality of the evidence, which followed the GRADE approach.15
Data analysis
Point estimates and 95% confidence intervals (95% CI) were calculated for the proportion of congenital anomalies reported among live births for each study. Where possible, we excluded spontaneous and induced abortions and stillbirths from the numerator and denominator for the estimate of congenital anomalies, consistent with current reporting conventions. Because of heterogeneity between studies, the overall prevalence of congenital anomalies was estimated by pooling data from each study using a DerSimonian-Laird random effects model16 following arcsine square-root transformation to stabilize the variance of the raw proportions,17 and subsequent back transformation to the original scale.18 Data derived from randomized trials were pooled together with data from observational studies using random effects analysis, consistent with recommended approaches for systematic reviews of adverse events.19 The following pre-planned subgroup analyses were conducted for the pooled prevalence estimate of congenital anomalies: trimester of cotrimoxazole exposure (first trimester versus second or third trimester), study design, and provision of folate supplementation (≥ 50% versus < 50% of the cohort). For outlier studies, we undertook a leave-one-out meta-analysis in which each study was dropped in turn to assess its influence on the overall pooled prevalence.20 Odds ratios (OR) and corresponding 95% CIs were calculated for data derived from case-control studies, and where appropriate, the data were pooled, also using random effects models. Data for secondary outcomes were not pooled because background prevalence rates are known to vary considerably between study settings. Heterogeneity was assessed using both the I2 and τ2 statistics.21 All analyses were conducted using STATA (version 12, www.stata.com) and GRADE Pro (www.gradeworkinggroup.org).
Results
Characteristics of included studies
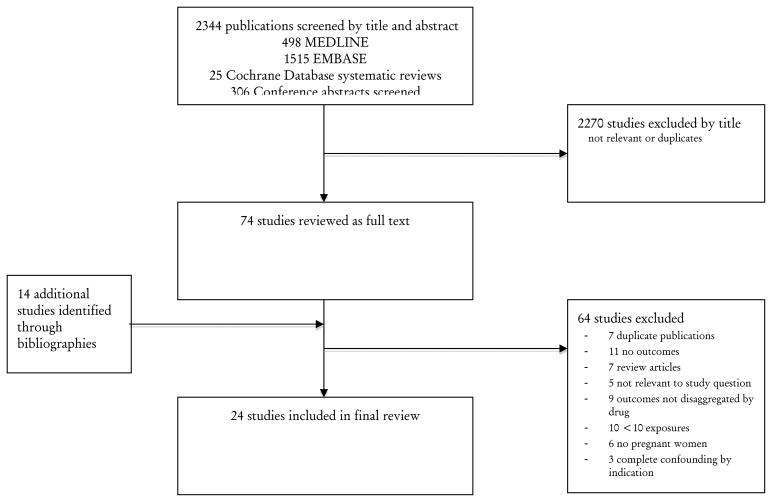
From a total of 2344 publications initially screened, 24 met the inclusion criteria and were taken through for full review (Figure 1).3,22–41 One paper reported the results of several studies in a single paper,26 one study reported data in two separate publications,30,42 and data from another, unpublished study, was reported in a review article.36 Two studies reported data from the Danish national pregnancy registry during overlapping time periods: one reported the risk of miscarriage among all births,22 the other reported the risk of congenital anomalies among live births.23 Four studies were carried out in sub-Saharan Africa (Benin,43 Malawi,44 Togo,45 and Zambia3), while the rest were carried out in high-income settings. Five studies reported outcomes among HIV-infected women.3,32,43–45 There were 4 surveillance studies, 6 randomized controlled trials (RCT), 5 case-control studies, and 9 cohort studies. The reporting period ranged from 197326 to 201245, with over half of the studies reporting data collected from 2002 onwards. Study characteristics are summarized in Table 1.
Figure 1.
Identification process for eligible studies
Table 1.
Study Characteristics
| Study | Design; setting |
Reporting period |
Overall study size |
Numbers receiving CTX |
Indication | HIV+ | Dose | Timing | Duration | Main drug provided |
Other drugs | Folate |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anderson et al, 201322 | Hospital registry cohort & Danish Fertility Registry; Denmark | 1997–2007 | 931,504 | 265 | UTI | 0 | NR | 1st Trimester | NR | TMP or TMP+SMX | Sulfamethizole given to 34/265 | NR |
| Anderson et al, 201323 | Danish Fertility Registry; Denmark | 1997–2005 | 521,267 | 402 | UTI (assumed) | 0 | NR | 12 weeks prior to pregnancy | NR | TMP or TMP+SMX | NR | 9 |
| Angelakis et al, 201324 | Retrospective cohort; France | 2006–2011 | 46 | 17 | Brucellosis | NR | NR | Throughout pregnancy | Variable (range 2 weeks – 6 months) | TMP+SMX | No | NR |
| Bailey et al, 198325 | RCT; New Zealand | 1980–1982 | 44 | 44 | Asymptomatic bacteriuria | NR | Either single dose CTX at 1.92g or 0.96g CTX BD (160 mg TM + 800mg SMX) for 5 days | <30 weeks gestation | Single dose or 5 days | TMP+SMX | No | No |
| Brumfitt & Pursell, 197326 | RCT; United Kingdom | 1973 | 155 | 120 +35 referrals | Bacteriuria | 0 | NR | Only 10<16 weeks pregnant; 35 referrals exposed at time of conception | Unclear | TMP+SMX | NR | NR |
| Carcopino et al, 200727 | Retrospective cohort; France | 1991–2005 | 53 | 22 | Q fever | NR | 320 mg TMP and 1600 mg SMX | Throughout pregnancy | Variable; mainly long-term >5 weeks | TMP+SMX | No | No |
| Colley et al, 198228 | Retrospective cohort; Australia | 1978–1981 | 7371 | 209* | Unclear | 0 | NR (most given “normal dose”) | Throughout pregnancy | NR | TMP+SMX | NR | NR |
| Czeizel et al, 200129 | Case-control study; Hungary | 1980–1996 | 61016 | 794 | Respiratory and UTI | 0 | 80 mg TMP + 400 mg SMX 2 tablets x 2/3 daily on day 1, then 1 tablet x 2/day | Throughout pregnancy | 4 days | TMP+SMX | Various | Cases 50% |
| Denoeud-Ndam et al, 201443 | RCT; Benin | 2009–2011 | 432 | 364 | HIV (malaria prophylaxis) | 432 | 160mg TMP + 800mg SMX | 2nd and 3rd trimester | Throughout pregnancy | TMP+SMX | Mefloquine (one arm, n=146); mefloquine, quinine or arthemeter–lumefantrine in case of malaria, depending on symptoms and levels of parasitaemia; antiretroviral therapy (32.7% AZT/3TC/EFV, 23.6% AZT/3TC/NVP, 18.1% D4T/3TC/NVP, 15.7% D4T/3TC/EFV) | 5 mg folic acid (all) |
| Dow et al, 201344 | RCT; Malawi | 2004–2009 | 1236 | 768 | HIV (malaria prophylaxis) | 1236 | 160mg TMP + 800mg SMX BD | 2nd and 3rd trimester | Throughout pregnancy | TMP+SMX | Antiretroviral therapy (225/768) | NR |
| Hernández-Díaz et al, 200030 | Case-control study; USA and Canada | 1976–1998 | 15319 | 66** | UTI | 0 | NR | −1 to +3 lunar months | NR | TMP+SMX | Unclear | 11% used daily peri-conceptional folic acid supplements |
| Hill et al, 198831 | Case-control study; United Kingdom | 1983 | 791*** | 42 | NR | NR | NR | 3 months pre-conception & 1st trimester | NR | TMP+sulpha drugs | NR | Unclear |
| Jungman et al, 200132 | Retrospective cohort; United Kingdom | 1994–1999 | 195 | 29 | HIV (prophylaxis) | 29 | NR | 1st Trimester | NR | TMP+SMX | No | NR |
| Khan et al, 200133 | Retrospective cohort; Saudi Arabia | 1983–1995 | 92 | 40 | Brucellosis | 0 | 160mg TMP + 800mg SMX BD | Throughout pregnancy | >=4 weeks | TMP+SMX (23) TMP+SMX + rifampicin (17) |
17 also received rifampicin | NR |
| Klement et al, 201445 | RCT; Togo | 2009–2012 | 264 | 126 (number analysed) | HIV (malaria prophylaxis) | 264 | 160mg TMP + 800mg SMX BD | 2nd and 3rd trimester | Throughout pregnancy | TMP+SMX | 300mg AZT or d4T, 3TC and NVP (depending on WHO Stage); malaria treatment where indicated | Yes (all) |
| Matok et al, 200934**** | Retrospective cohort; Israel | 1998–2007 | 84823 | 346 | “primarily” UTI | NR | NR | 1st trimester | Mean 7.4 days for all DHRI | TMP+SMX | 2 women methotrexate only, 1 sulfasalazine only | NR |
| Meijer et al, 200535 | Case-Control study; Netherlands | 1997–2002 | 2217 | 15 | Unclear | NR | NR | 1st Trimester | Unclear | TMP | 7 TMP only, 2 TMP+sulfoxamide, 3 sulfasalazine | ~24% all cases and controls |
| Michigan Medicaid surveillance study, 200336 | Surveillance; USA | Unclear | 2296 | 2296 | Unclear | NR | NR | NR | NR | TMP+SMX | NR | NR |
| Roushan, et al 201137 | Retrospective case series; Iran | 2000–2010 | 19 | 14 | Brucellosis | NR | NR | Throughout pregnancy | 2 months | TMP+SMX | Rifampicin (all women) | NR |
| Santos et al, 201138 | Case-control (prospectively collected data); Canada | 1998–2003 | 63338 | 214 | Unclear | NR | NR | Throughout pregnancy | Variable | TMP+SMX | NR | NR |
| Valentini et al, 200939 | Retrospective hospital case review; Italy | 2009 (published) | 76 | 76 | Toxoplasmosis | NR | 160mg TMP + 800mg SMX BD | 2nd and 3rd trimester (Start at least after week 14, and stop 2 weeks before delivery) | Variable – up to 24 weeks | TMP+SMX | Spiramycin (all women) | Yes (all) |
| Walter et al, 20063 | Cohort nested within an RCT; Zambia | 2001–2004 | 255 | 67 | HIV (prophylaxis) | 67 | 80mg TMP + 400mg SMX BD | Delayed until 2nd trimester | Ongoing; dependent on CD4 | TMP+SMX | Chloroquine (2002) then sulfadoxine-pyrimethamine (2003) ART | 800 mg (all women) |
| Wen et al, 200840 | Retrospective cohort; Canada | 1980–2000 | 74807 | DHRI: 11386 TMP-SMX: 12546 | NR (any exposure but mostly UTI) | NR | NR | Pre-conception period and throughout pregnancy | NR | TMP+SMX | No | NR |
| Yaris et al, 200441 | Toxicology Information and Follow-up Service; Turkey | 1999–2004 | 511 | 11 | UTI | NR | TM-SMX 160–800 mg for 7–10 days (2 cases with Gentamicin) | 1st trimester | 7–10 days | TMP+SMX | Gentamicin in 2 cases | NR (unlikely) |
CTX, cotrimoxazole; 3TC, lamivudine; ART, antiretroviral therapy; AZT, zidovudine; BD, twice daily; DHRI dihydrofolate reductase inhibitors; NR, not reported; NVP, nevirapine; RCT Randomised control trial; TMP+SMX, cotrimoxazole (TMP trimethoprim; SMX sulfamethoxizole); UTI, urinary tract infection
127 additional patients given sulfamethizole alone
Trimethoprim, triamterene, and sulfasalazine. Exact numbers not given
Cases only
Includes fetal anomalies diagnosed via prenatal ultrasound
Congenital anomalies
Prevalence of congenital anomalies
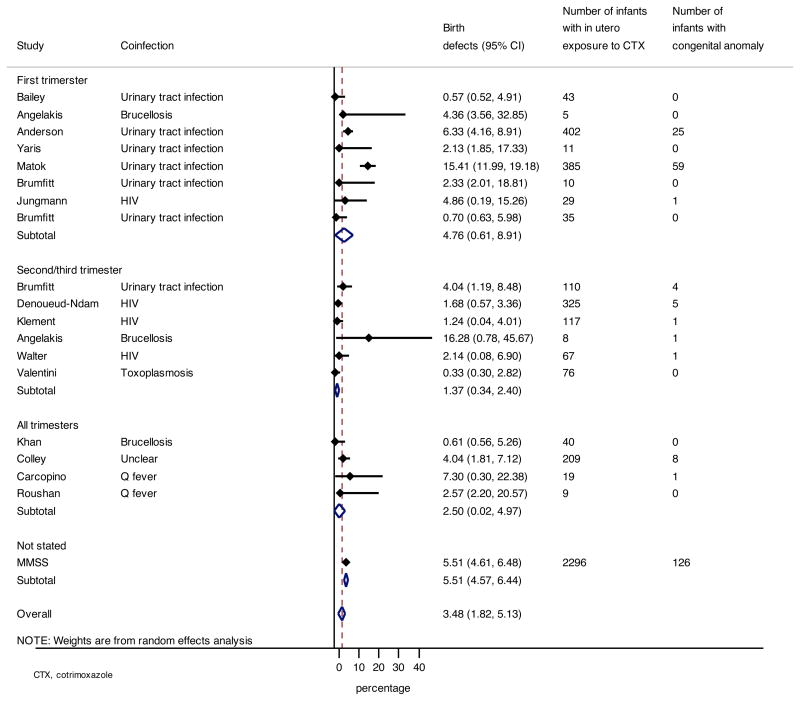
Sixteen studies reported 232 infants with congenital anomalies among 4196 women receiving cotrimoxazole during pregnancy,3,23–28,32–34,36,37,39,41,43,45 summarized in Table 2. The remaining eight studies were not included in this analysis for the following reasons: five were case-control studies,29–31,38,46 while the remaining three studies reported only data regarding secondary outcomes.22,44,40 The prevalence of congenital anomalies ranged from 0.3% (95% CI 0.3–2.8%) to 16.3% (95% CI 0.8–45.7%), with an overall pooled prevalence of 3.5% (95% CI 1.8–5.1%; I2 84.9%, τ2 0.03).34 The pooled prevalence was higher for studies that included pregnant women receiving cotrimoxazole during the first trimester of pregnancy (4.8%, 95%CI 0.6–8.9%) compared to studies in which there were no first trimester exposures (1.4%, 95%CI 0.3–2.4%). These differences were, however, not found to be statistically significant (P=0.1). These data are summarized in Figure 2. When comparing the reported prevalence of congenital anomalies by study design, the pooled prevalence was higher for population surveillance studies (7.8%, 95%CI 3.6–11.9%) compared to RCTs (1.4%, 95%CI 0.5–2.4%), prospective cohort studies (2.1%, 95%CI 0–5.6%) or retrospective cohorts (2.0%, 95%CI 0.1–3.6%). The pooled prevalence of birth defects in infants in studies that included HIV-infected pregnant women3,32,43,45 (1.7%, 95%CI 0.6–2.7%) was lower compared to the overall prevalence in all women.
Table 2.
Congenital anomalies (Case control studies not included)
| Study | Trimester | Number of live births to women receiving CTX (N=4196) | Number of infants with congenital anomaly (N=232) | Number of infants with neural tube congenital anomaly (N=32) | Type of anomaly (all reported) |
|---|---|---|---|---|---|
| Anderson et al, 201323 | T1 | 402 | 25* | 1 | Limb defects Urinary tract defects (UTD) Orofacial defects Cardiovascular defects (CVD) Neural tube defects (NTD) |
| Angelakis et al, 201324 | T1 | 5 | 0 | 0 | -- |
| Angelakis et al, 201324 | T2/3 | 8 | 1 | 0 | Bilateral renal agenesis |
| Bailey et al, 198325 | T1/T2 | 43 | 0 | 0 | -- |
| Brumfitt & Pursell, 197326 | T1 | 10 | 0 | 0 | -- |
| Brumfitt & Pursell, 197326 | T1 | 35 | 0 | 0 | -- |
| Brumfitt & Pursell, 197326 | T2/3 | 110 | 4** | Cleft lip, hypospadias, Robin syndrome, extra
digits All cases at T2 |
|
| Carcopino et al, 200727 | NS | 19 | 1 | 0 | Potter’s syndrome |
| Colley et al, 198228 | T1/2/3 | 209 | 8*** | 1 | Patent ductus arteriosis Atrial septal defect Hypospadias Dislocated hip Metatarsus varus Cavernous haemangioma Other |
| Denoueud-Ndam et al, 201443 | T2/3 | 325 | 5**** | 0***** | Clubfoot Umbilical hernia Hydrocephaly |
| Jungman et al, 200132 | T1 | 29 | 1 | 1 | NTD, hydrocephalus |
| Khan et al, 200133 | NS | 40 | 0 | 0 | -- |
| Klement et al, 201445 | T2/3 | 117 | 1 | 0 | Polydactyly |
| Matok et al, 200934 | T1 | 385 | 59 | 29 | 29 NTD 13 CVD 4 UTD |
| Michigan Medicaid surveillance study, 200336 | NS | 2296 | 126 | -- | 37 CVD |
| Roushan et al, 201137 | T1-3 | 9 | 0 | 0 | -- |
| Valentini et al, 200939 | T2/3 | 76 | 0 | 0 | -- |
| Walter et al, 20063 | T2/3 | 67 | 1 | NR | NR |
| Yaris et al, 200441 | T1 | 11 | 0 | 0 | NR |
CTX, cotrimoxazole; CVD, cardiovascular defects; G, gestation; NR, not reported; NTD, neural tube defects; T, trimester; UTD, urinary tract defects
Only major malformations included
All events were associated with T2 exposure
All events were associated with T2/3 exposure
An additional malformation was reported among a stillborn infant
There was one intra uterine fetal death diagnosed with encephalocele and ventral hernia
Figure 2.
Pooled prevalence of infant congenital anomalies by trimester
We conducted a sensitivity analysis in which the pooled prevalence was assessed after dropping each study in turn in order to determine the degree of influence of any single study on the overall pooled prevalence estimate of congenital anomalies. In this analysis, the pooled prevalence was reduced from 3.5% (1.8–5.1%) to 2.6% (95% 1.2–4.0) when one study34 was dropped from the analysis (Supplementary Table 1). This study differed from the other studies in two notable ways: first, fetal anomalies were diagnosed in utero; second, around half of the cohort were Bedouins in Israel, a community in which there is increased consanguinity.47 Incidence of birth defects were reported to be higher among the Bedouin community during the reporting period of this study.48 These factors may have led to both a higher ascertainment of congenital anomalies, and a higher background prevalence of anomalies in this cohort.
Among the 16 studies reporting on the prevalence of congenital anomalies, three studies reported 31 infants with neural tube defects, giving a crude prevalence of 0.7% (95%CI 0.5–1.0%). These data were also dominated by the study described above,34 which contributed 29 of 31 neural tube defects.
Odds of congenital anomalies
Four case-control studies, reported in five articles,29–31,42,46 provided data on the OR of congenital anomalies comparing cotrimoxazole exposure among cases and controls. There was no statistically significant difference in the risk of overall congenital anomalies (2 studies; OR 0.6, 95%CI 0.1–3.4), with high heterogeneity between studies (I2 84%, τ2 1.2). An increased risk was reported for neural tube defects (1 study; OR 3.4, 95%CI 1.1–10.3;), cardiovascular defects (1 study; OR 2.9, 95%CI 1.6–5.5), and oral clefts (2 studies; OR 2.0, 95% CI 1.2–3.4; I2 0%, τ2 0) but not urinary tract defects (1 study; OR 0.9, 95% CI 0.2–3.9).
Secondary outcomes
Five studies provided data on maternal toxicity.26,27,39,43,45 Of 714 women exposed to cotrimoxazole during pregnancy in these studies (490 with HIV infection), 31 (4.3%) experienced an adverse drug reaction; the majority of events were mild with only 4 events (0.6%) resulting in treatment discontinuation. One study provided data on neonatal jaundice, reporting one case out of 67 exposures.3 Other reported birth outcomes included stillbirths (6 studies), spontaneous abortions (6 studies), small for gestational age (7 studies), and pre-term birth (6 studies). Data were not pooled due to the limited data reported for these outcomes, differing background population rates, and risk of confounding by indication. No cases of kernicterus were reported. These outcomes are summarized in Supplementary Tables 2 and 3.
Assessment of methodological quality
Risk of bias was considered to be moderate to high. Seven studies did not directly ascertain cotrimoxazole use, 15 studies did not assess the potential influence of confounders, 14 studies used retrospective designs, 9 studies did not disaggregate outcomes by trimester of exposure, 17 studies did not report on folate use, and 7 studies were at risk of confounding by indication for secondary outcomes (Supplementary Table 4). The risk of publication bias was considered to be high, considering that cohorts among whom adverse outcomes occurred are more likely to be documented and published. This was not formally assessed due to the small number of identified studies. Overall, the GRADE assessment determined that the quality of the evidence contributing to the assessment of prevalence and odds of congenital anomalies was very low. This information is summarized in Supplementary Tables 5 and 6.
Conclusions
Cotrimoxazole has been commonly prescribed for over forty years for the treatment of a wide range of infections, and has been recommended as life-saving prophylaxis by the WHO for all HIV-infected individuals with low CD4 cell count, including pregnant women. Despite its long history of widespread use, this review found very limited evaluable data on maternal and infant outcomes associated with cotrimoxazole exposure during pregnancy. While some studies included in this review suggested that cotrimoxazole exposure in pregnancy was associated with congenital abnormalities, the overall pooled prevalence was not significantly higher than the reported rates in the general population.49 In the United States, the prevalence of congenital anomalies is 2.7%50 while in sub-Saharan Africa, the reported prevalence ranges from 0.4% to 3.7%.51 Evidence from case-control studies suggests a potential increased risk of certain specific congenital anomalies, including neural tube defects. The crude prevalence of neural tube defects was 0.7%, which is higher than that reported among the general population in the United States (0.04–0.06% before regular folic acid fortification),52 United Kingdom (0.14%)53 and South Africa (0.36%);54 however, most of the defects were reported by one study in which prenatal ultrasound screening was standard and that included a population with increased consanguinity34. Thus, the poor quality of the data prevents any definitive conclusions from being drawn. Nevertheless, this review found some reassuring evidence of cotrimoxazole safety. Although the data were limited, maternal treatment limiting adverse events were rare. There was no evidence of excessive rates of infant jaundice associated with cotrimoxazole exposure in pregnancy, although only 1 study reported on neonatal jaundice.
A previous systematic assessment of cotrimoxazole safety in pregnancy, carried out in 2006, took a broad approach that considered any sulfonamide exposure, concluding that there was mixed evidence about safety in pregnancy; overall estimates of risk were not calculated.9 We limited our review to cotrimoxazole, but did include HIV-uninfected cohorts as well as exposure to cotrimoxazole for both treatment and for prophylaxis of various infectious diseases. Our search strategy and inclusion criteria allowed for the identification of over 4,000 exposures in randomized trials and observational cohort studies that could contribute to estimating prevalence of congenital anomalies, with additional information provided by case-control studies. Confidence intervals were calculated for outcomes of individual studies and for pooled estimates in order to reflect the level of uncertainty around the prevalence estimates. In order to compensate for the limited number of studies reporting outcomes specifically for cotrimoxazole prophylaxis, we included studies in which pregnant women received cotrimoxazole for the treatment of various infections. The duration of drug exposure and the indication for its use – including for infections known to be associated with some of the secondary outcomes under assessment – differed considerably between these studies. Although the primary outcome of birth defects was not found to be influenced by infection status in sensitivity analyses, secondary outcomes should nevertheless be interpreted with particular caution given the risk of confounding by indication. Other methodological issues of concern included the retrospective nature of many studies, the inadequate reporting of timing and duration of cotrimoxazole exposure, the limited reporting of folate use and other potential confounders, and the likelihood of publication bias favoring the documentation and reporting of adverse outcomes. These limitations resulted in the quality of the evidence being rated as very low. Further studies are needed to improve judgment about the safety of cotrimoxazole in pregnancy.
Despite the widespread use of cotrimoxazole prophylaxis in HIV-infected individuals, including pregnant women, an important limitation of our review is the limited data found on birth outcomes in HIV-infected pregnant women receiving long-term cotrimoxazole prophylaxis. The majority of studies included in this review reported on short-term cotrimoxazole use for the treatment of various infectious diseases, such as urinary tract infections, brucellosis, toxoplasmosis or Q fever. Less than a quarter of data contributing to the prevalence assessment came from HIV-infected women, and only one of these studies (29 exposures) reported on first-trimester cotrimoxazole use; this study suggested that exposure to the combination of antiretroviral drugs and folate antagonists was associated with an increased risk of congenital abnormalities. In this study, of 32 women receiving folate antagonists in the first trimester, 3 were not exposed to cotrimoxazole but to other antifolates (pyrimethamine, carbamazepine) and only 13 women received both antiretroviral and anti-folate drugs (and the study did not delineate whether all 13 were exposed to cotrimoxazole or to the other anti-folates).27 Almost all data on birth defects for HIV-infected women were from Africa, where concomitant nutritional deficiencies may be more common than in resource-rich countries.
This review highlights several areas for future research. First, improved surveillance is critical to gather data on cotrimoxazole exposure during pregnancy, as is the case for a number of drugs commonly used in the management of HIV/AIDS, notably efavirenz. This review found few reports from high HIV-burden, resource-limited settings, where cotrimoxazole prophylaxis is likely to be of most benefit. The lack of screening for congenital abnormalities and the high rate of unattended deliveries in these settings may change the programmatic implications of the results reported by this review. It will be critical for pregnancy outcome surveillance to include an evaluation of exposure to cotrimoxazole as well as antiretroviral drugs. Pregnancy outcome surveillance is being reinforced in several countries with the support of WHO and major donors, and the findings from this work will help inform future guidance. More data are needed for all important maternal and infant outcomes when using cotrimoxazole for prophylaxis in HIV-infected pregnant women, in particular in settings of high malaria prevalence, with outcomes disaggregated by trimester and duration of cotrimoxazole exposure, and where possible, reporting of relevant concomitant exposures (e.g., smoking, folate and other nutritional supplementation, use of other drugs). Finally, cotrimoxazole inhibits folic acid synthesis, and two studies included in this review suggested that periconceptional daily folic acid supplementation may reduce cotrimoxazole’s potential teratogenic effect.29,42 However, concomitant folate use may reduce the antibacterial effect of cotrimoxazole, which warrants further study.
In conclusion, the findings of this review support continued recommendations to provide cotrimoxazole prophylaxis to HIV-infected pregnant women. As with the use of any drug in pregnancy, the benefits of the drug need to be weighed against its potential risks. It is crucial that data collection on maternal and infant outcomes is improved to better assess the safety of cotrimoxazole use during pregnancy. Because of the substantial mortality reduction benefits associated with cotrimoxazole use in HIV-infected individuals with low immunity1,2 and the particular vulnerability of HIV-infected pregnant women to diseases potentially preventable by cotrimoxazole such as malaria, continued recommendations for cotrimoxazole as a priority intervention for HIV-infected pregnant women is warranted.
Supplementary Material
Acknowledgments
This work was partially funded by a grant from the Bill and Melinda Gates Foundation. JJ received salary support from NICHD K23HD070760 during the preparation of this manuscript. The conclusions and views expressed in this article are those of the authors and do not necessarily reflect those of their respective organizations. The authors would like to thank Marco Vitoria for comment on an earlier draft.
Footnotes
There are no conflicts of interest.
References
- 1.Anglaret X, Chene G, Attia A, et al. Early chemoprophylaxis with trimethoprim-sulphamethoxazole for HIV-1-infected adults in Abidjan, Cote d’Ivoire: a randomised trial. Cotrimo-CI Study Group. Lancet. 1999;353(9163):1463–8. doi: 10.1016/s0140-6736(98)07399-1. [DOI] [PubMed] [Google Scholar]
- 2.Suthar AB, Granich R, Mermin J, Van Rie A. Effect of cotrimoxazole on mortality in HIV-infected adults on antiretroviral therapy: a systematic review and meta-analysis. Bull World Health Organ. 2012;90(2):128C–38C. doi: 10.2471/BLT.11.093260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walter J, Mwiya M, Scott N, et al. Reduction in preterm delivery and neonatal mortality after the introduction of antenatal cotrimoxazole prophylaxis among HIV-infected women with low CD4 cell counts. J Infect Dis. 2006;194(11):1510–8. doi: 10.1086/508996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anonymous. Recommendations for a public health approach. WHO; Geneva: 2006. Guidelines on co-trimoxazole prophylaxis for HIV-related infections among children, adolescents and adults. [PubMed] [Google Scholar]
- 5.Mandell GLSM. Antimicrobial agents. In: Gilman AG, Rall TW, Nies AS, Taylor P, editors. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 8. New York: Pergamon Press; 1990. pp. 1047–1064. [Google Scholar]
- 6.Scholl TO, Johnson WG. Folic acid: influence on the outcome of pregnancy. The American journal of clinical nutrition. 2000;71(5 Suppl):1295S–303S. doi: 10.1093/ajcn/71.5.1295s. [DOI] [PubMed] [Google Scholar]
- 7.Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. MRC Vitamin Study Research Group. Lancet. 1991;338(8760):131–7. [PubMed] [Google Scholar]
- 8.Reid DW, Caille G, Kaufmann NR. Maternal and transplacental kinetics of trimethoprim and sulfamethoxazole, separately and in combination. Canadian Medical Association journal. 1975;112(13 Spec):67–72. [PMC free article] [PubMed] [Google Scholar]
- 9.Forna F, McConnell M, Kitabire FN, et al. Systematic review of the safety of trimethoprim-sulfamethoxazole for prophylaxis in HIV-infected pregnant women: implications for resource-limited settings. AIDS Rev. 2006;8(1):24–36. [PubMed] [Google Scholar]
- 10.Gea Briggs., editor. Drugs in pregnancy and lactation. 5. Williams and Wilkens; 1988. [Google Scholar]
- 11.Townsend CL, Willey BA, Cortina-Borja M, Peckham CS, Tookey PA. Antiretroviral therapy and congenital abnormalities in infants born to HIV-infected women in the UK and Ireland, 1990–2007. AIDS. 2009;23(4):519–24. doi: 10.1097/QAD.0b013e328326ca8e. [DOI] [PubMed] [Google Scholar]
- 12.http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/017377s068s073lbl.pdf
- 13.http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/017377s067lbl.pdf
- 14.Anonymous. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; [Accessed 26 April, 2014]. Available at http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. [Google Scholar]
- 15.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 17.Freeman MFTJ. Transformations related to the angular and the square root. Ann Inst Stat Mathematics. 1950;21:607–611. [Google Scholar]
- 18.Miller J. The inverse of the Freeman-Tukey double arcsine transformation. Am Stat. 1978;32:138. [Google Scholar]
- 19.Golder S, Loke YK, Bland M. Meta-analyses of adverse effects data derived from randomised controlled trials as compared to observational studies: methodological overview. PLoS Med. 2011;8(5):e1001026. doi: 10.1371/journal.pmed.1001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Viechtbauer WCM. Outlier and influence diagnostics for meta-analysis. Research synthesis methods. 2010;1:112–125. doi: 10.1002/jrsm.11. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andersen JT, Petersen M, Jimenez-Solem E, et al. Trimethoprim use in early pregnancy and the risk of miscarriage: a register-based nationwide cohort study. Epidemiol Infect. 2013;141(8):1749–55. doi: 10.1017/S0950268812002178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andersen JT, Petersen M, Jimenez-Solem E, et al. Trimethoprim Use prior to Pregnancy and the Risk of Congenital Malformation: A Register-Based Nationwide Cohort Study. Obstet Gynecol Int. 2013;2013:364526. doi: 10.1155/2013/364526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Angelakis E, Million M, D’Amato F, et al. Q fever and pregnancy: disease, prevention, and strain specificity. Eur J Clin Microbiol Infect Dis. 2013;32(3):361–8. doi: 10.1007/s10096-012-1750-3. [DOI] [PubMed] [Google Scholar]
- 25.Bailey RR, Bishop V, Peddie BA. Comparison of single dose with a 5-day course of co-trimoxazole for asymptomatic (covert) bacteriuria of pregnancy. Aust N Z J Obstet Gynaecol. 1983;23(3):139–41. doi: 10.1111/j.1479-828x.1983.tb00562.x. [DOI] [PubMed] [Google Scholar]
- 26.Brumfitt W, Pursell R. Trimethoprim-sulfamethoxazole in the treatment of bacteriuria in women. J Infect Dis. 1973;128(Suppl):657–65. doi: 10.1093/infdis/128.supplement_3.s657. [DOI] [PubMed] [Google Scholar]
- 27.Carcopino X, Raoult D, Bretelle F, Boubli L, Stein A. Managing Q fever during pregnancy: the benefits of long-term cotrimoxazole therapy. Clin Infect Dis. 2007;45(5):548–55. doi: 10.1086/520661. [DOI] [PubMed] [Google Scholar]
- 28.Colley DKJ, Gibson G. A study on the use in pregnancy of co-trimoxazole and sulphamethizole. Australian J Pharmacy. 1982:570–575. [Google Scholar]
- 29.Czeizel AE, Rockenbauer M, Sorensen HT, Olsen J. The teratogenic risk of trimethoprim-sulfonamides: a population based case-control study. Reprod Toxicol. 2001;15(6):637–46. doi: 10.1016/s0890-6238(01)00178-2. [DOI] [PubMed] [Google Scholar]
- 30.Hernandez-Diaz S, Werler MM, Walker AM, Mitchell AA. Folic acid antagonists during pregnancy and the risk of birth defects. N Engl J Med. 2000;343(22):1608–14. doi: 10.1056/NEJM200011303432204. [DOI] [PubMed] [Google Scholar]
- 31.Hill L, Murphy M, McDowall M, Paul AH. Maternal drug histories and congenital malformations: limb reduction defects and oral clefts. J Epidemiol Community Health. 1988;42(1):1–7. doi: 10.1136/jech.42.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jungmann EM, Mercey D, DeRuiter A, et al. Is first trimester exposure to the combination of antiretroviral therapy and folate antagonists a risk factor for congenital abnormalities? Sex Transm Infect. 2001;77(6):441–3. doi: 10.1136/sti.77.6.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khan MY, Mah MW, Memish ZA. Brucellosis in pregnant women. Clin Infect Dis. 2001;32(8):1172–7. doi: 10.1086/319758. [DOI] [PubMed] [Google Scholar]
- 34.Matok I, Gorodischer R, Koren G, Landau D, Wiznitzer A, Levy A. Exposure to folic acid antagonists during the first trimester of pregnancy and the risk of major malformations. Br J Clin Pharmacol. 2009;68(6):956–62. doi: 10.1111/j.1365-2125.2009.03544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meijer WM, de Walle HE, Kerstjens-Frederikse WS, de Jong-van den Berg LT. Folic acid sensitive birth defects in association with intrauterine exposure to folic acid antagonists. Reproductive toxicology (Elmsford, NY) 2005;20(2):203–7. doi: 10.1016/j.reprotox.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 36.Sivojelezova A, Einarson A, Shuhaiber S, Koren G. Trimethoprim-sulfonamide combination therapy in early pregnancy. Can Fam Physician. 2003;49:1085–6. [PMC free article] [PubMed] [Google Scholar]
- 37.Roushan MR, Baiani M, Asnafi N, Saedi F. Outcomes of 19 pregnant women with brucellosis in Babol, northern Iran. Trans R Soc Trop Med Hyg. 2011;105(9):540–2. doi: 10.1016/j.trstmh.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 38.Santos F, Sheehy O, Perreault S, Ferreira E, Berard A. Exposure to anti-infective drugs during pregnancy and the risk of small-for-gestational-age newborns: a case-control study. BJOG. 2011;118(11):1374–82. doi: 10.1111/j.1471-0528.2011.03041.x. [DOI] [PubMed] [Google Scholar]
- 39.Valentini P, Annunziata ML, Angelone DF, et al. Role of spiramycin/cotrimoxazole association in the mother-to-child transmission of toxoplasmosis infection in pregnancy. Eur J Clin Microbiol Infect Dis. 2009;28(3):297–300. doi: 10.1007/s10096-008-0612-5. [DOI] [PubMed] [Google Scholar]
- 40.Wen SW, Zhou J, Yang Q, Fraser W, Olatunbosun O, Walker M. Maternal exposure to folic acid antagonists and placenta-mediated adverse pregnancy outcomes. CMAJ. 2008;179(12):1263–8. doi: 10.1503/cmaj.080859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yaris F, Kadioglu M, Kesim M, et al. Urinary tract infections in unplanned pregnancies and fetal outcome. Eur J Contracept Reprod Health Care. 2004;9(3):141–6. doi: 10.1080/13625180400007744. [DOI] [PubMed] [Google Scholar]
- 42.Hernandez-Diaz S, Werler MM, Walker AM, Mitchell AA. Neural tube defects in relation to use of folic acid antagonists during pregnancy. Am J Epidemiol. 2001;153(10):961–8. doi: 10.1093/aje/153.10.961. [DOI] [PubMed] [Google Scholar]
- 43.Denoeud-Ndam L, Zannou DM, Fourcade C, et al. Cotrimoxazole prophylaxis versus mefloquine intermittent preventive treatment to prevent malaria in HIV-infected pregnant women: two randomized controlled trials. J Acquir Immune Defic Syndr. 2014;65(2):198–206. doi: 10.1097/QAI.0000000000000058. [DOI] [PubMed] [Google Scholar]
- 44.Dow A, Kayira D, Hudgens MG, et al. The effect of cotrimoxazole prophylactic treatment on malaria, birth outcomes, and postpartum CD4 count in HIV-infected women. Infectious diseases in obstetrics and gynecology. 2013;2013:340702. doi: 10.1155/2013/340702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klement E, Pitche P, Kendjo E, et al. Effectiveness of co-trimoxazole to prevent Plasmodium falciparum malaria in HIV-positive pregnant women in sub-Saharan Africa: an open-label, randomized controlled trial. Clin Infect Dis. 2014;58(5):651–9. doi: 10.1093/cid/cit806. [DOI] [PubMed] [Google Scholar]
- 46.Meijer WM, de Walle HE, Kerstjens-Frederikse WS, de Jong-van den Berg LT. Folic acid sensitive birth defects in association with intrauterine exposure to folic acid antagonists. Reprod Toxicol. 2005;20(2):203–7. doi: 10.1016/j.reprotox.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 47.Vardi-Saliternik R, Friedlander Y, Cohen T. Consanguinity in a population sample of Israeli Muslim Arabs, Christian Arabs and Druze. Annals of human biology. 2002;29(4):422–31. doi: 10.1080/03014460110100928. [DOI] [PubMed] [Google Scholar]
- 48.Silberstein E, Silberstein T, Elhanan E, Bar-Droma E, Bogdanov-Berezovsky A, Rosenberg L. Epidemiology of cleft lip and palate among Jews and Bedouins in the Negev. Isr Med Assoc J. 2012;14(6):378–81. [PubMed] [Google Scholar]
- 49.Anonymous. March of dimes birth defects foundation white plains. New york: 2006. Global report on birth defects. Http://www.Marchofdimes.Com/glue/files/birthdefectsexecutivesummary.Pdf. [Google Scholar]
- 50.Correa A, Cragan JD, Kucik JE, et al. Reporting birth defects surveillance data 1968–2003. Birth defects research Part A, Clinical and molecular teratology. 2007;79(2):65–186. doi: 10.1002/bdra.20350. [DOI] [PubMed] [Google Scholar]
- 51.Orenstein LA, Orenstein EW, Teguete I, et al. Background rates of adverse pregnancy outcomes for assessing the safety of maternal vaccine trials in sub-Saharan Africa. PloS one. 2012;7(10):e46638. doi: 10.1371/journal.pone.0046638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Centers for Disease C and Prevention. Spina bifida and anencephaly before and after folic acid mandate--United States, 1995–1996 and 1999–2000. MMWR Morbidity and mortality weekly report. 2004;53(17):362–5. [PubMed] [Google Scholar]
- 53.Rankin J, Pattenden S, Abramsky L, et al. Prevalence of congenital anomalies in five British regions, 1991–99. Archives of disease in childhood Fetal and neonatal edition. 2005;90(5):F374–9. doi: 10.1136/adc.2003.047902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Venter PA, Christianson AL, Hutamo CM, Makhura MP, Gericke GS. Congenital anomalies in rural black South African neonates--a silent epidemic? South African medical journal. 1995;85(1):15–20. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.