Abstract
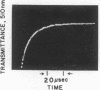
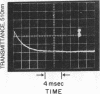
The intensely chromophoric intramolecular coordination complex formed between arsanilazotyrosine-248 and the active site zinc atom of azocarboxypeptidase A (Johansen, J. T. & Vallee, B. L. (1971) Proc. Nat. Acad. Sci. USA 68, 2532-2535) is a spectrokinetic probe of catalytic events. The interconversion of the azoTyr-248-Zn complex and its constituents is measured by stopped-flow pH and temperature-jump methods. The rate of interconversion, 64,000 sec-1, is orders of magnitude faster than that of the catalytic step itself (about 0.01-100 sec-1). Rapidly turned over peptide and ester substrates disrupt the azoTyr-248-Zn complex before hydrolysis occurs. As a consequence, formation of azoTyr-248, substrate binding, and catalysis can all be monitored while catalysis is actually in progress. The results of these dynamic studies specify a course of catalytic events, different from those postulated based on x-ray structure analysis. If azoTyr-248 is displaced, the direction is opposite to the inward movement postulated on the basis of x-ray studies and is not unique to induction by substrates, since rapid changes in pH also result in analogous spectral changes. AzoTyr-248 carboxypeptidase has all the features which are essential for mechanistic studies: (1) It is enzymatically active; (2) the spectra of the metal complex differ characteristically from those of its constituents; (3) it responds dynamically to environmental factors; and (4) the response time of the probe itself is much more rapid than is required for the measurement of the catalytic step. These combined kinetic and spectral properties of the metal complex render it a powerful spectrokinetic probe to visualize and discern microscopic details of the catalytic process.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auld D. S., Holmquist B. Carboxypeptidase A. Differences in the mechanisms of ester and peptide hydrolysis. Biochemistry. 1974 Oct 8;13(21):4355–4361. doi: 10.1021/bi00718a018. [DOI] [PubMed] [Google Scholar]
- Auld D. S., Vallee B. L. Kinetics of carboxypeptidase A. II. Inhibitors of the hydrolysis of oligopeptides. Biochemistry. 1970 Feb 3;9(3):602–609. doi: 10.1021/bi00805a022. [DOI] [PubMed] [Google Scholar]
- French T. C., Yu N. T., Auld D. S. Relaxation spectra of proteinases. Isomerizations of carboxypeptidase A (Cox) and (Anson). Biochemistry. 1974 Jul 2;13(14):2877–2882. doi: 10.1021/bi00711a016. [DOI] [PubMed] [Google Scholar]
- Johansen J. T., Vallee B. L. Conformations of arsanilazotyrosine-248 carboxypeptidase A alpha, beta, gamma, comparison of crystals and solution. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2006–2010. doi: 10.1073/pnas.70.7.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen J. T., Vallee B. L. Differences between the conformation of arsanilazotyrosine 248 of carboxypeptidase A in the crystalline state and in solution. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2532–2535. doi: 10.1073/pnas.68.10.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen J. T., Vallee B. L. Environment and conformation dependent sensitivity of the arsanilazotyrosine-248 carboxypeptidase A chromophore. Biochemistry. 1975 Feb 25;14(4):649–660. doi: 10.1021/bi00675a001. [DOI] [PubMed] [Google Scholar]
- Lipscomb W. N. Enzymatic activities of carobxypeptidase A's in solution and in crystals. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3797–3801. doi: 10.1073/pnas.70.12.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscomb W. N., Hartsuck J. A., Reeke G. N., Jr, Quiocho F. A., Bethge P. H., Ludwig M. L., Steitz T. A., Muirhead H., Coppola J. C. The structure of carboxypeptidase A. VII. The 2.0-angstrom resolution studies of the enzyme and of its complex with glycyltyrosine, and mechanistic deductions. Brookhaven Symp Biol. 1968 Jun;21(1):24–90. [PubMed] [Google Scholar]
- Lipscomb W. N., Reeke G. N., Jr, Hartsuck J. A., Quiocho F. A., Bethge P. H. The structure of carboxypeptidase A. 8. Atomic interpretation at 0.2 nm resolution, a new study of the complex of glycyl-L-tyrosine with CPA, and mechanistic deductions. Philos Trans R Soc Lond B Biol Sci. 1970 Feb 12;257(813):177–214. doi: 10.1098/rstb.1970.0020. [DOI] [PubMed] [Google Scholar]
- Quiocho F. A., McMurray C. H., Lipscomb W. N. Similarities between the conformation of arsanilazotyrosine 248 of carboxypeptidase A in the crystalline state and in solution. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2850–2854. doi: 10.1073/pnas.69.10.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spilburg C. A., Bethune J. L., Vallee B. L. The physical state dependence of carboxypeptidase Aalpha and Agamma kinetics. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3922–3926. doi: 10.1073/pnas.71.10.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee B. L., Riordan J. F., Johansen J. T., Livingston D. M. Spectro-chemical probes for protein conformation and function. Cold Spring Harb Symp Quant Biol. 1972;36:517–531. doi: 10.1101/sqb.1972.036.01.066. [DOI] [PubMed] [Google Scholar]