Abstract
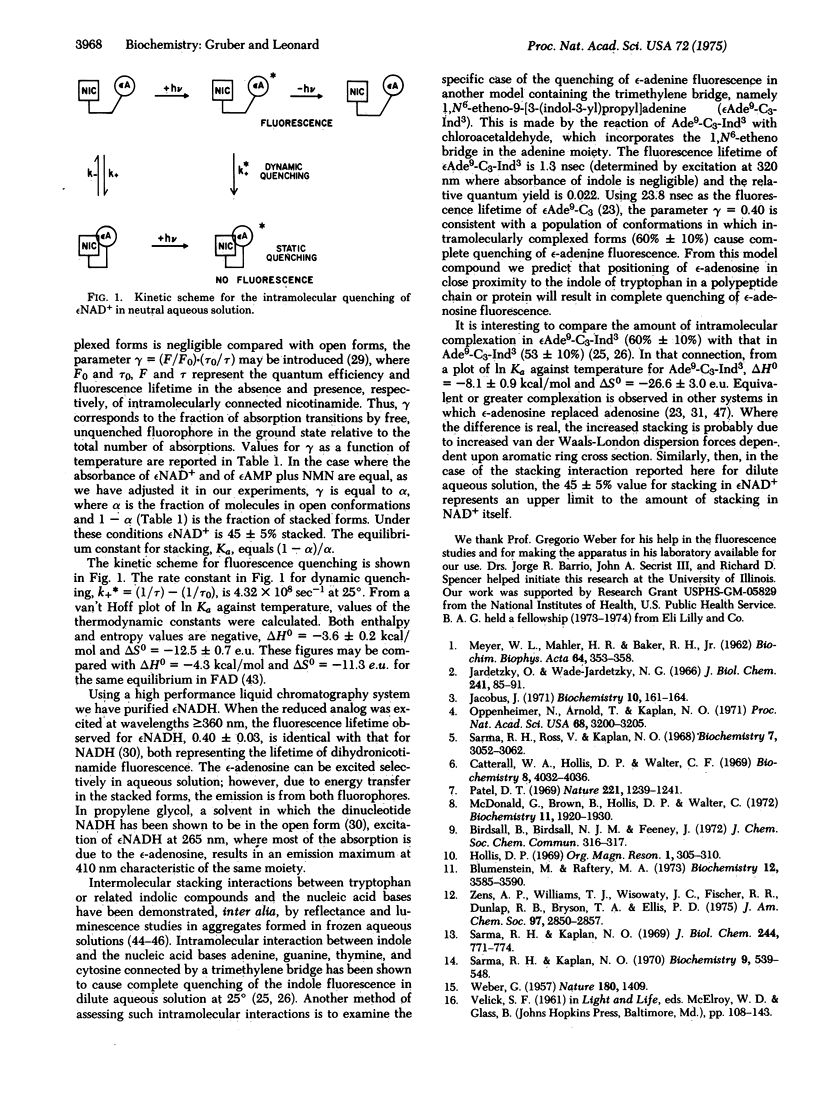
For nicotinamide 1,N6-ethenoadenine dinucleotide (epsilonNAD+), the fluorescent analog of NAD+, in neutral aqueous solution the quantum yield has been determined to be 0.028 and the fluorescent lifetime, 2.1 nsec. Simultaneous determination of quantum yields and lifetimes of epsilonNAD+ and of the "half molecule" epsilonAMP allows the calculation of the percentage of stacked and open conformations of the dinucleotide. At 25 degrees in neutral aqueous solution there is 45 +/- 5% of stacked forms. The value of the fluorescent impurities, especially those containing the epsilon-adenosine moiety, and a purification procedure using high performance liquid chromatography was devised to obtain fluorescently homogeneous preparations. In order to study the effect on epsilon-adenosine fluorescence caused by the possible close proximity of a tryptophan in a polypeptide chain or protein, we have prepared 1,N6-etheno-9-[3-(indol-3-yl)propyl]adenine (epsilonAde9-C3-Ind3), a model compound in which indole is used as a neutral substitute for tryptophan. Fluorescence studies on epsilonAde9-C3-Ind3 show that the formation of an intramolecular complex results in complete quenching of the epsilon-adenine fluorescence. It is therefore predictable that positioning of the epsilon-adenosine of any fluorescent coenzyme moiety (e.q., epsilonATP, epsilonADP) in close proximity to a tryptophan in a protein will result in complete fluorescence quenching of the former.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrio J. R., Leonard N. J. Specific and fluorescent modifications of cytidine. J Am Chem Soc. 1973 Feb 21;95(4):1323–1328. doi: 10.1021/ja00785a051. [DOI] [PubMed] [Google Scholar]
- Barrio J. R., Secrist J. A., 3rd, Leonard N. J. A fluorescent analog of nicotinamide adenine dinucleotide. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2039–2042. doi: 10.1073/pnas.69.8.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrio J. R., Tolman G. L., Leonard N. J., Spencer R. D., Weber G. Flavin 1, N 6 -ethenoadenine dinucleotide: dynamic and static quenching of fluorescence. Proc Natl Acad Sci U S A. 1973 Mar;70(3):941–943. doi: 10.1073/pnas.70.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenstein M., Raftery M. A. Natural abundance 13C nuclear magnetic resonance spectra of nicotinamide adenine dinucleotide and related nucleotides. Biochemistry. 1973 Sep 11;12(19):3585–3590. doi: 10.1021/bi00743a001. [DOI] [PubMed] [Google Scholar]
- Brun F., Toulmé J. J., Hélène C. Interactions of aromatic residues of proteins with nucleic acids. Fluorescence studies of the binding of oligopeptides containing tryptophan and tyrosine residues to polynucleotides. Biochemistry. 1975 Feb 11;14(3):558–563. doi: 10.1021/bi00674a015. [DOI] [PubMed] [Google Scholar]
- Catterall W. A., Hollis D. P., Walter C. F. Nuclear magnetic resonance study of the conformation of nicotinamide--adenine dinucleotide and reduced nicotinamide--adenine dinucleotide in solution. Biochemistry. 1969 Oct;8(10):4032–4036. doi: 10.1021/bi00838a021. [DOI] [PubMed] [Google Scholar]
- Greenfield J. C., Leonard N. J., Gumport R. I. Nicotinamide 3,N4-ethenocytosine dinucleotide, an analog of nicotinamide adenine dinucleotide. Synthesis and enzyme studies. Biochemistry. 1975 Feb 25;14(4):698–706. doi: 10.1021/bi00675a009. [DOI] [PubMed] [Google Scholar]
- Jacobus J. Conformation of pyridine dinucleotides in solution. Biochemistry. 1971 Jan 5;10(1):161–164. doi: 10.1021/bi00777a023. [DOI] [PubMed] [Google Scholar]
- Jardetzky O., Wade-Jardetzky N. G. The conformation of pyridine dinucleotides in solution. J Biol Chem. 1966 Jan 10;241(1):85–91. [PubMed] [Google Scholar]
- Jeck R., Wilhelm G. Wechselwirkungen der nichtfunktionellen Coenzymbindungsstelle in Dehydrogenasen mit (Nicotinamid-ribofuranosyl)-(omega-(adenin-9-yl)-n-alkyl)-pyrophosphaten. Justus Liebigs Ann Chem. 1973;3:531–543. doi: 10.1002/jlac.197319730317. [DOI] [PubMed] [Google Scholar]
- Lee C. Y., Everse J. Studies on the properties of 1,N 6 -ethenoadenine derivatives of various coenzymes. Arch Biochem Biophys. 1973 Jul;157(1):83–90. doi: 10.1016/0003-9861(73)90392-5. [DOI] [PubMed] [Google Scholar]
- Luisi P. L., Baici A., Bonner F. J., Aboderin A. A. Relationship between fluorescence and conformation of epsilonNAD+ bound to dehydrogenases. Biochemistry. 1975 Jan 28;14(2):362–368. doi: 10.1021/bi00673a024. [DOI] [PubMed] [Google Scholar]
- McDonald G., Brown B., Hollis D., Walter C. Some effects of environment on the folding of nicotinamide-adenine dinucleotides in aqueous solutions. Biochemistry. 1972 May 9;11(10):1920–1930. doi: 10.1021/bi00760a029. [DOI] [PubMed] [Google Scholar]
- Montenay-Garestier T., Hélène C. Molecular interactions between tryptophan and nucleic acid components in frozen aqueous solutions. Nature. 1968 Mar 2;217(5131):844–845. doi: 10.1038/217844a0. [DOI] [PubMed] [Google Scholar]
- Montenay-Garestier T., Hélène C. Reflectance and luminescence studies of molecular complex formation between tryptophan and nucleic acid components in frozen aqueous solutions. Biochemistry. 1971 Jan 19;10(2):300–306. doi: 10.1021/bi00778a016. [DOI] [PubMed] [Google Scholar]
- Mutai K., Gruber B. A., Leonard N. J. Synthetic spectroscopic models. Intramolecular stacking interactions between indole and connected nucleic acid bases. Hypochromism and fluorescence. J Am Chem Soc. 1975 Jul 9;97(14):4095–4104. doi: 10.1021/ja00847a038. [DOI] [PubMed] [Google Scholar]
- Oppenheimer N. J., Arnold L. J., Kaplan N. O. A structure of pyridine nucleotides in solution. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3200–3205. doi: 10.1073/pnas.68.12.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D. J. 220 MHz proton magnetic resonance spectrum of NADH. Nature. 1969 Mar 29;221(5187):1239–1241. doi: 10.1038/2211239a0. [DOI] [PubMed] [Google Scholar]
- Sarma R. H., Kaplan N. O. 220 MHz nuclear magnetic resonance spectra of oxidized and reduced pyridine dinucleotides. J Biol Chem. 1969 Feb 25;244(4):771–774. [PubMed] [Google Scholar]
- Sarma R. H., Kaplan N. O. High frequency nuclear magnetic resonance study of the M and P helices of reduced pyridine dinucleotides. Biochemistry. 1970 Feb 3;9(3):539–548. doi: 10.1021/bi00805a013. [DOI] [PubMed] [Google Scholar]
- Sarma R. H., Ross V., Kaplan N. O. Investigation of the conformation of beta-diphosphopyridine nucleotide (beta-nicotinamide-adenine dinucleotide) and pyridine dinucleotide analogs by proton magnetic resonance. Biochemistry. 1968 Sep;7(9):3052–3062. doi: 10.1021/bi00849a005. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Levitzki A. Molecular basis of negative co-operativity in rabbit muscle glyceraldehyde-3-phosphate dehydrogenase. J Mol Biol. 1974 Feb 5;82(4):547–561. doi: 10.1016/0022-2836(74)90248-4. [DOI] [PubMed] [Google Scholar]
- Secrist J. A., 3rd, Barrio J. R., Leonard N. J. Attachment of a fluorescent label to 4-thiouracil and 4-thiouridine. Biochem Biophys Res Commun. 1971 Dec 3;45(5):1262–1270. doi: 10.1016/0006-291x(71)90154-9. [DOI] [PubMed] [Google Scholar]
- Secrist J. A., 3rd, Barrio J. R., Leonard N. J., Weber G. Fluorescent modification of adenosine-containing coenzymes. Biological activities and spectroscopic properties. Biochemistry. 1972 Sep 12;11(19):3499–3506. doi: 10.1021/bi00769a001. [DOI] [PubMed] [Google Scholar]
- Spencer R. D., Weber G., Tolman G. L., Barrio J. R., Leonard N. J. Species responsible for the fluorescence of 1:N6-ethenoadenosine. Eur J Biochem. 1974 Jun 15;45(2):425–429. doi: 10.1111/j.1432-1033.1974.tb03566.x. [DOI] [PubMed] [Google Scholar]
- Tolman G. L., Barrio J. R., Leonard N. J. Chloroacetaldehyde-modified dinucleoside phosphates. Dynamic fluorescence quenching and quenching due to intramolecular complexation. Biochemistry. 1974 Nov 19;13(24):4869–4878. doi: 10.1021/bi00721a001. [DOI] [PubMed] [Google Scholar]
- Wang A. H., Dammann L. G., Barrio J. R., Paul I. C. The crystal and molecular structure of a derivative of 1,N6-ethenoadenosine hydrochloride. Dimensions and molecular interactions of the fluorescent epsilon-adenosine (epsilon-ado) system. J Am Chem Soc. 1974 Feb 20;96(4):1205–1212. doi: 10.1021/ja00811a038. [DOI] [PubMed] [Google Scholar]
- Ward D. C., Horn T., Reich E. Fluorescence studies of nucleotides and polynucleotides. 3. Diphosphopyridine nucleotide analogues which contain fluorescent purines. J Biol Chem. 1972 Jun 25;247(12):4014–4020. [PubMed] [Google Scholar]
- Warshaw M. M., Tinoco I., Jr Absorption and optical rotatory dispersion of six dinucleoside phosphates. J Mol Biol. 1965 Aug;13(1):54–64. doi: 10.1016/s0022-2836(65)80079-1. [DOI] [PubMed] [Google Scholar]
- Warshaw M. M., Tinoco I., Jr Optical properties of sixteen dinucleoside phosphates. J Mol Biol. 1966 Sep;20(1):29–38. doi: 10.1016/0022-2836(66)90115-x. [DOI] [PubMed] [Google Scholar]
- Woenckhaus C., Scherr D. Eigenschaften des Coenzymanalogen Bis-nicotinamid-dinucleotid. Hoppe Seylers Z Physiol Chem. 1973 Jan;354(1):53–59. doi: 10.1515/bchm2.1973.354.1.53. [DOI] [PubMed] [Google Scholar]
- Zens A. P., Williams T. J., Wisowaty J. C., Fisher R. R., Dunlap R. B., Bryson T. A., Ellis P. D. Nuclear magnetic resonance studies on pyridine dinucleotides. II. Solution conformational dynamics of nicotinamide adenine dinucleotide and nicotinamide mononucleotide as viewed by proton T1 measurements. J Am Chem Soc. 1975 May 14;97(10):2850–2857. doi: 10.1021/ja00843a040. [DOI] [PubMed] [Google Scholar]


