Abstract
In this work, we sought to rationally design membrane active peptides that are triggered by low pH to form macromolecular-sized pores in lipid bilayers. Such peptides could have broad utility in biotechnology and in nanomedicine as cancer therapeutics or drug delivery vehicles that promote release of macromolecules from endosomes. Our approach to rational design was to combine the properties of a pH-independent peptide, MelP5, which forms large pores allowing passage of macromolecules, with the properties of two pH-dependent membrane active peptides, pHlip and GALA. We created two hybrid sequences, MelP5_Δ4 and MelP5_Δ6 by using the distribution of acidic residues on pHlip and GALA as a guide to insert acidic amino acids into the amphipathic helix of MelP5. We show that the new peptides bind to lipid bilayers and acquire secondary structure in a pH-dependent manner. The peptides also destabilize bilayers in a pH-dependent manner, such that lipid vesicles release the small molecules ANTS/DPX at low pH only. Thus, we were successful in designing pH-triggered pore-forming peptides. However, no macro-molecular release was observed under any conditions. Therefore, we abolished the unique macromolecular poration properties of MelP5 by introducing pH-sensitivity into its sequence. We conclude that the properties of pHlip, GALA and MelP5 are additive, but only partially so. We propose that this lack of additivity is a limitation in the rational design of novel membrane active peptides, and that high-throughput approaches to discovery will be critical for continued progress in the field.
INTRODUCTION
Many potential applications of membrane active peptides have been discussed in the literature (1–8). Yet, their utility has been limited, partly because many such peptides come from natural sources and have not been engineered with particular applications in mind (9, 10). We recently embarked on a project to synthetically evolve a potent pore-forming peptide (11). We reported that an analog of the bee venom lytic peptide melittin, MelP5, possesses the unique ability to assemble into macromolecule-sized, equilibrium pores under conditions where natural membrane active sequences are inactive (12). Unlike the parent peptide melittin, which forms small, transient pores under most conditions (1, 13, 14), MelP5 forms pores that persist for hours and allow the passage of macromolecules across bilayers, including dextrans (10 kDa) and chymotrypsin (24 kDa), even at very low peptide concentrations (11, 12).
A question arises if MelP5 can be engineered such that its unique macromolecular poration activity can be controlled by pH. Of particular interest would be the design of a peptide that is not membrane active at pH 7, but interacts with membranes and forms macromolecule-sized equilibrium pores at pH ~5.0. A peptide with this property would be useful for multiple biomedical applications. For example, it would be useful in the generic delivery of macromolecules into cells via endocytosis where endosomal acidification would lead to release of endocytosed macromolecular cargo into the cytosol. pH-sensitive membrane active peptides could also be targeted to tumor cells, where membrane permeabilization would be triggered by the decreased pH that occurs in the vicinity of solid tumors (15). While a peptide that forms macromolecular pores with pH-sensitive activity currently does not exist, there are known membrane active peptides with pH-sensitive membrane binding, insertion and permeabilization that guided the work described here. One such peptide, called pHLIP, is a monomeric random coil at neutral pH, but inserts into membranes as a monomeric, membrane-spanning α-helix upon acidification, with an apparent pKa of ~6.0 (16, 17). pHLIP does not cause membrane permeabilization. A compositionally similar peptide, called GALA, is likewise a random coil at neutral pH but assembles into highly active, but small, membrane-spanning pores with an apparent pKa of ~5.5 (18, 19).
Each of these pH-sensitive, membrane active peptides has acidic glutamate and aspartate residues interspersed into an otherwise hydrophobic sequence (Figure 1). Each folds into an α-helix in membranes when partitioning and folding become favorable at low pH. Similarly, MelP5 folds into an amphipathic helix when bound to membranes, although MelP5 does so in a pH-independent manner. To approach our goal of designing pH-sensitive macromolecular pore forming peptides, we sought to combine the macromolecular pore-formation of MelP5 with the pH-sensitive membrane activity of pHLIP and GALA. Here we report on the rational design of two such peptides, and on the investigation of their membrane activities over a pH range between 3 and 7.
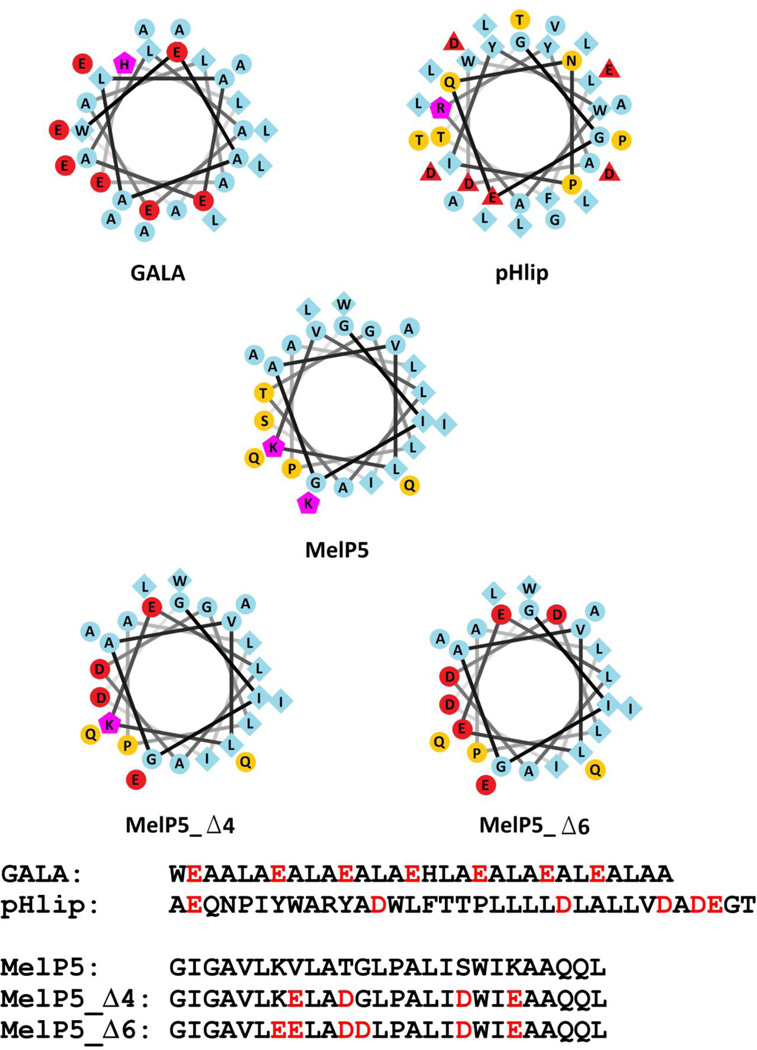
Figure 1.
Helical wheel projection diagrams of GALA, pHLIP, MelP5, MelP5_Δ4 and MelP5_Δ6. Residues colored blue are hydrophobic, yellow are hydrophilic, red are anionic at neutral pH and purple are cationic at neutral and low pH. Below are the sequences of the five peptides. The red letters signify the acidic residues in GALA and pHlip, and the acidic residues inserted into the sequence of MelP5 to make MelP5_Δ4 (4 substitutions) and MelP5_Δ6 (6 substitutions).
MATERIALS and METHOD
Reagents
1-palmitoyl-2-oleoly-sn-3-glycero-phosphocholine (POPC) was purchased from Avanti Polar lipids as a lyophilized powder and dissolved in chloroform at 25 mg/ml for use. MelP5 used in this study, as well as MelP5_Δ4 and MelP5_Δ6, were synthesized by Biosynthesis, Inc. and were solubilized in methanol. Melittin was purchased as a lyophilized powder from Sigma-Aldrich and dissolved in methanol. Biotin-dextran-(10kD)-TAMRA and Avidin-Alexafluor488 were purchased from Invitrogen. All other reagents were purchased from Sigma-Aldrich.
Buffer Preparation
All buffers were made using millipore purified water. Sodium phosphate buffers were prepared at 10 mM sodium phosphate with or without 100 mM potassium chloride at both pH 6 and pH 7. Sodium Acetate buffers were prepared at 10 mM sodium acetate concentration with or without 100 mM potassium chloride at pH 5, pH 4.5, pH 4, pH 3.5 and pH 3. Buffer for the ANTS/DPX experiment was created by adding 12.5 mM ANTS, 45 mM DPX, 5 mM HEPES and 20 mM potassium chloride. Buffer for the FRET-leakage assay was created by adding 1 mg/ml of Biotin-Dextran (10kD)-TAMRA to either pH 7 sodium phosphate buffer or pH 4 sodium acetate buffer described above.
Vesicle Preparation
POPC vesicles were prepared by standard methods (20, 21). Lipids in chloroform were dispensed into glass vial using glass syringes, dried under nitrogen for at least 15 minutes, and then dried completely under high vacuum for at least 3 hours. After drying, lipids were resuspended in buffer. The suspension was subjected to 10 cycles of freezing in liquid nitrogen followed by thawing in a bath of room temperature water, and then the suspension was extruded 10 times through 0.1 µm pore Nuclepore polycarbonate filters. The lipid concentration was then measured using a modified version of the Stewart assay (22).
Binding Assay
Binding assays were performed with fluorescence titration (23) using 10 µM peptide solution in one of the pH buffers and titrating up to 10 mM lipid. Intensities at 335 nm were obtained for each lipid concentration and normalized to the intensity measured in the absence of lipid. To enable correction for vesicle-dependent light scattering effects, as described elsewhere, (23), we also measured the normalized intensity of 10 µM free tryptophan (which does not bind to bilayers) at 335 nm at the same lipid concentrations. To obtain values for the mole fraction partition coefficient (Kx) the normalized and corrected intensity data were fit to the equation:
where I(L) is the normalized, corrected fluorescence at lipid concentration L, I∞-1 is the maximum fluorescence enhancement, and 55.3 M is the molar concentration of water. Partition coefficients, Kx, were converted to free energies using the Gibbs equation:
Circular Dichroism Spectroscopy
Samples for Circular Dichroism (CD) Spectroscopy were prepared by diluting the peptides from methanol into sodium phosphate or sodium acetate buffers without potassium chloride at various pH values to a final peptide concentration of 100 µM. After the ellipticity was recorded, vesicles were added to a final concentration of 1 mM and the ellipticity was recorded again. These data were collected with a scanning rate of 0.5 nm/sec.
ANTS/DPX, small molecule leakage assay
To test for small pores in synthetic bilayers, vesicles were prepared with encapsulated ANTS/DPX solution at either pH 4 or pH 7. These vesicle solutions were diluted to 1 mM lipid concentration for leakage experiments. The samples were excited at 360 nm and the emission was collected at 530 nm over a period of about 200–300 seconds. After about 50 seconds, varying amounts of the peptides were added to the solution. The emission intensity was recorded for up to 150 seconds at which time 10 µL of 10% Triton X-100 was added to lyse the vesicles. Fractional leakage from the vesicles was calculated using the equation
where Ipeptide is the signal after adding the peptide, Ifinal is the signal after adding the triton and Ibackground is the signal without adding anything extra to the solution.
FRET-based, large molecule leakage assay
Large molecule leakage was assayed by preparing solutions of vesicles containing biotin-Dextran (10kD)-TAMRA. These vesicles were diluted to 1 mM lipid concentration and 10 µM Avidin-Alexafluor488 was added to the solution. The samples were excited at 495 nm, the excitation maximum for Alexafluor488, and the emission was recorded at 588, close to the excitation maximum of TAMRA. The signal would only be seen, therefore, if the biotin-Dextran (10kD)-TAMRA molecules were close enough, or bound, to the Avidin-Alexafluor488 to cause FRET. Thus, FRET occurs only if one, or both, of the macromolecules can cross the membrane. The background signal was recorded for about 50 seconds, at which time peptides were added to a final ratio of 1:100 peptide:lipid. The signal was then recorded until about 150 seconds, after which time 10 µL of 10% Triton-100× was added to fully lyse the vesicles. The fractional leakage was analyzed using Equation 2 as shown above.
RESULTS
Peptide Design
The membrane interactions and folding of amphipathic -helical peptides are driven by partitioning into bilayers, due to hydrophobicity, and by the coupling of partitioning and folding, due to amphipathicity (24). We have argued that the unique structural and functional properties of MelP5 are dominated by the nearly ideal amphipathic helix along its length (12). The pH-sensitive peptides GALA and pHLIP are likewise hydrophobic sequences that are long enough to span a lipid bilayer and fold into an α-helix. However, these peptides are prevented from binding and folding, at neutral pH, by the fact that there are multiple aspartate or glutamate residues distributed along the putative hydrophobic helix. When fully charged, these acidic residues decrease the interfacial hydrophobicity and disfavor folding due to repulsive interactions in the putative helix. For both GALA and pHLIP, a decrease in pH shifts the equilibrium towards the membrane-bound, α-helical state. At a characteristic pH of 5–6, the free energy of partitioning and folding can overcome the repulsion of the electrostatic groups and the peptide will insert into bilayers and fold into helices.
Here we sought to engineer the same kind of pH sensitivity into analogs of MelP5, while also preserving MelP5’s unique pore-forming properties. To do this we modified either 4 or 6 residues of MelP5 to aspartate or glutamate as shown in Figure 1. Our goal was to create an appropriate arrangement of charges on the MelP5 backbone that was similar to GALA and pHLIP. We applied several functional design principles. First, and most importantly, we wished to maximize the number of i to i+3, i to i+4, and i to i+7 helical spacings to maximize the pH sensitivity of the helical secondary structure. Second, as in GALA, we wanted the charged residues to be distributed somewhat asymmetrically on the helical wheel, and to overlap the polar face of MelP5, to promote pore formation. Third, we wanted the charges to be well distributed along the helix length, but not on the helical ends where the charge effects might be smaller. Fourth, we did not want the residues of MelP5 that were replaced with acidic residues to include multiple hydrophobic residues (leucine, isoleucine and tryptophan) because this would decrease the overall hydrophobicity and membrane binding, even at low pH. Indeed, a protonated or uncharged carboxyl group is still polar and inserts somewhat unfavorably into membranes (24, 25)). Fifth, residues thought to be critical for the structure of MelP5 (e.g. Alal10,Pro14 and Ala23) were not altered. Using these principles, we designed two sequences to test. MelP5_Δ4 has four substitutions (V8,T11,S18 and K20 for glutamate or aspartate). This variant has 3 pairs of acidic residues that have helical spacings, and it only loses one hydrophobic residue, valine 8. MelP5_Δ6 has 6 substitutions, (the same four as above, plus K7 and G12 for glutamate and aspartate). This variant has six pairs of acidic residues with helical spacings. The sequences and the helical wheels of the parent peptides and the designed peptide analogs are shown in Figure 1.
Binding and Secondary Structure formation
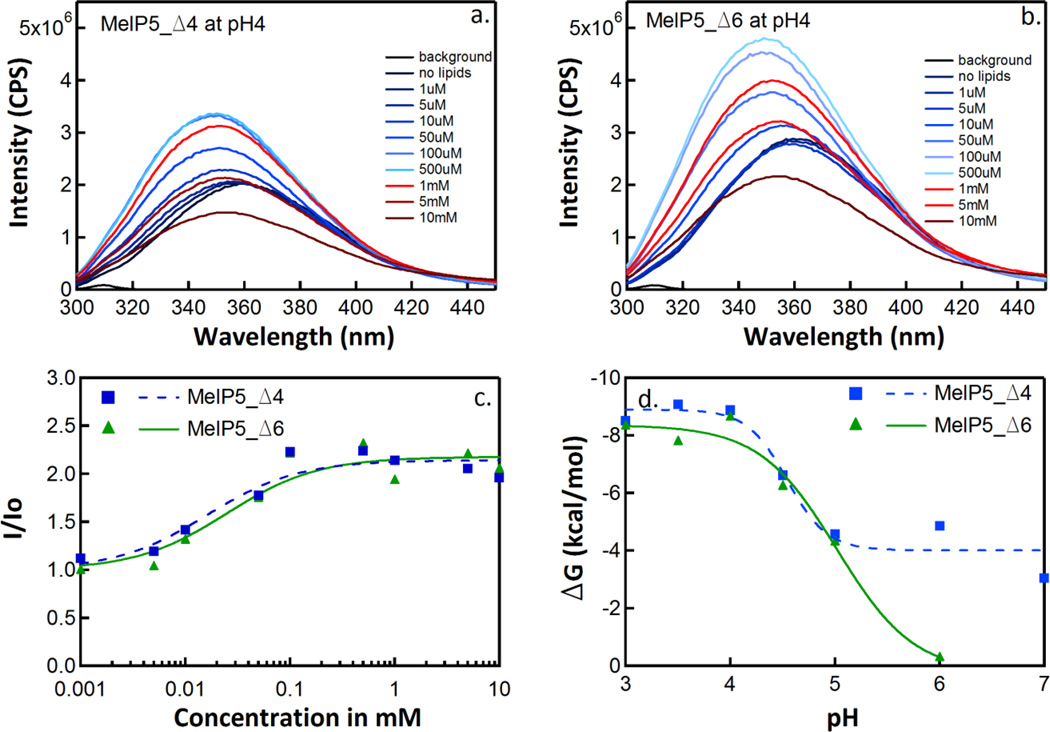
We monitored binding of MelP5_Δ4 and MelP5_Δ6 to phosphatidylcholine vesicles as a function of pH using fluorescence titration at various pH values. Example spectra at pH 4 are shown in Figure 2a and 2b. Upon lipid titration, the tryptophan fluorescence emission spectrum shifts to shorter wavelengths and increases in intensity due to binding. At higher lipid concentrations intensities decrease due to light scattering, an effect that we correct for in the manner described in Materials and Methods. Spectra are converted into binding isotherms as in Figure 2c, and from these curves we obtain partition coefficients Kx as described elsewhere (26). From Kx, we calculate ΔG = −RTln(Kx). The pH-dependence of the free energy of partitioning of MelP5_Δ4 and MelP5_Δ6 are shown in Figure 2d. At neutral pH, MelP5_Δ6 binds very weakly to membranes, while MelP5_Δ4 binds measurably, consistent with its more favorable overall hydrophobicity and fewer charge pairs with helical spacing. As the pH is decreased, both peptides bind much more strongly, such that by pH 4 both variants bind as well to bilayers as MelP5, which binds very strongly to membranes (11, 12). The apparent pKa values for the binding curves are about 4.6 for MelP5_Δ4 and 4.8 for MelP5_Δ6.
Figure 2.
Peptide binding by tryptophan fluorescence titration. This figure shows the results of binding experiments performed with the peptides MelP5_Δ4 and MelP5_Δ6. Panels a and b show tryptophan emission spectra after titration of 10 µM peptide with increasing amounts of POPC vesicles at pH 4. Panel c shows the lipid dependence of the change in fluorescence intensity (I/Io) at 335 nm, at pH 4, after correction for light scattering effects (see text). Data such as those in panel c were fit to Equation 1 to obtain mole fraction partitioning coefficients for each peptide/pH combination. Panel d shows the change in free energy of partitioning with respect to pH.
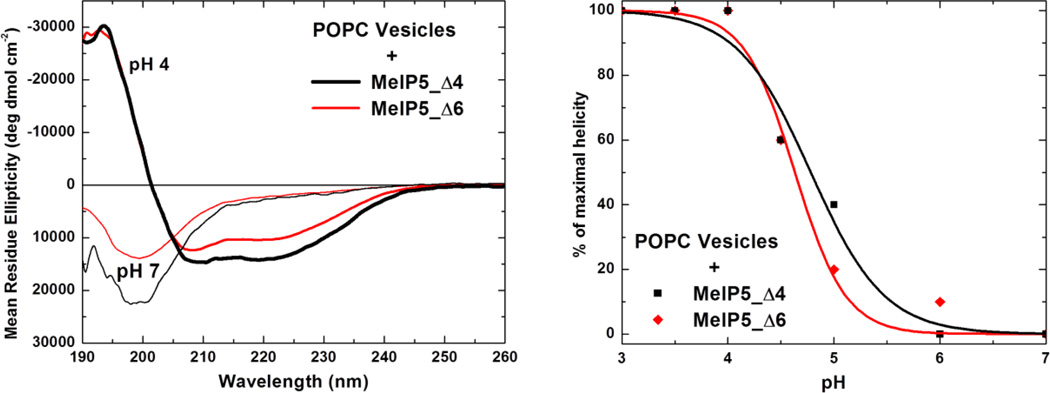
In order to determine if the peptides also acquired secondary structure in a pH-controlled manner, circular dichroism spectra were collected for these samples in the presence of 1 mM POPC vesicles at various pH values. Figure 3a shows example CD spectra for MelP5_Δ4 and MelP5_Δ6 at pH 7 and pH 4 in the presence of vesicles. The peptides have random coil secondary structure at pH 7, as shown by the large minimum at 200 nm, but become α-helical at pH 4, as shown by the typical α-helix minima at 208 nm and 222 nm. In figure 3b we show the normalized α-helical content, calculated from the ellipticity at 222 nm, as a function of pH. These data follow the same trend with respect to pH as the binding data shown in Figure 2, demonstrating that binding and structure formation are coupled. At a characteristic pH value between 4.6 and 4.8, both peptides undergo cooperative structural transitions from random coil to α-helix.
Figure 3.
Secondary structure of MelP5_Δ4 and MelP5_Δ6. Left: The spectra of 100 µM MelP5_Δ4 and MelP5_Δ6 in the presence of 1 mM POPC lipid vesicles, at neutral pH and at acidic pH. At pH 4, the spectra indicate α-helical structure, whereas at pH 7 the spectra are characteristic of random coil structure. Such measurements were carried out as a function of pH and the results are plotted in the right panel. The fraction helix is calculated from the ellipticity at 222 nm and is normalized to the maximal value, which is obtained at pH 4.
The helical content of the two analogs at low pH is roughly 50%, which is only a little less than the helical content of melittin or MelP5 in membranes (11). Taken together, binding and CD data show that we have successfully engineered pH sensitivity into the sequence of MelP5, and that the new peptides interact with membranes and fold into α-helices at around pH 5, a range where useful biological activity can be triggered.
Pore Forming Activity in Model Membranes
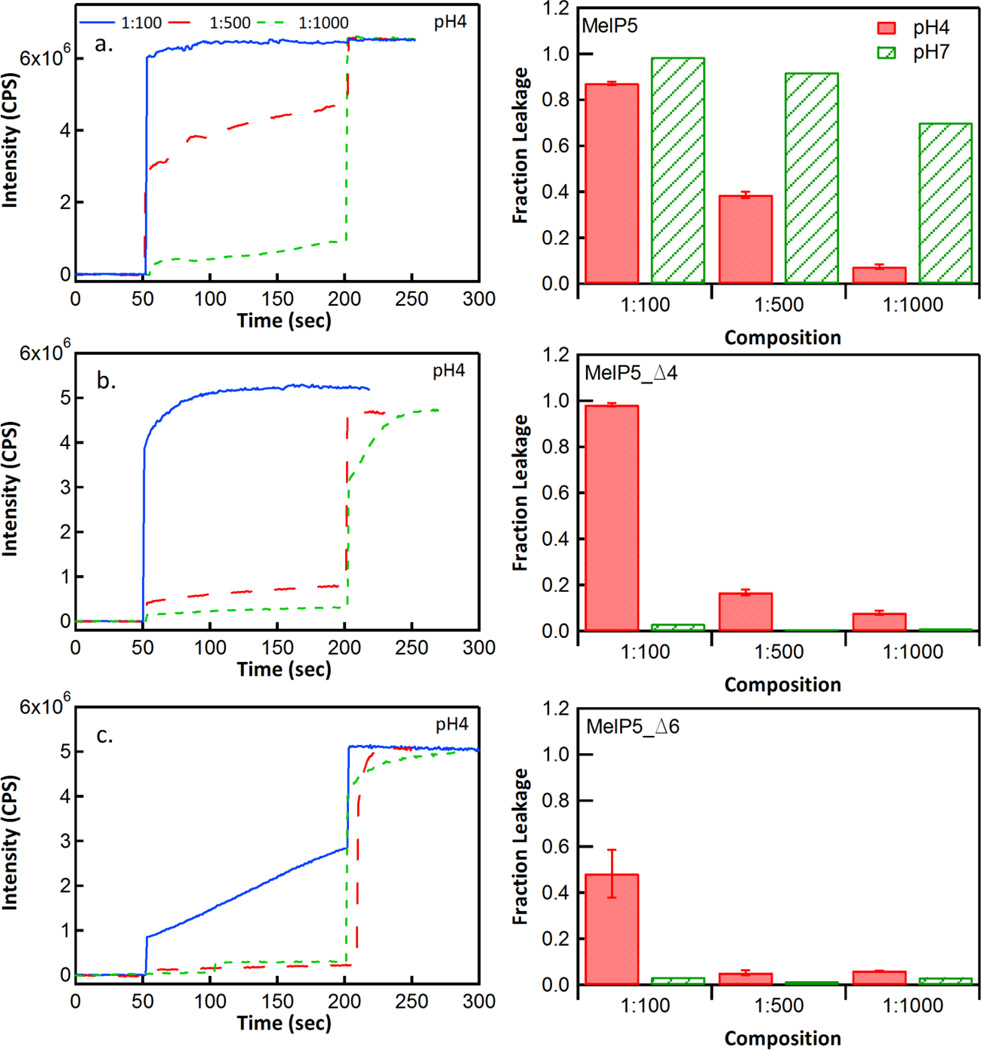
We performed vesicle leakage assays to characterize the membrane activity and pore forming properties of MelP5_Δ4 and MelP5_Δ6 as functions of pH. First, we measured leakage of the small molecule probes, ANTS/DPX, which are ~350 Da each. As shown in Figure 4 and elsewhere (12), Melp5 is very potent and releases more than 50% of the entrapped molecules at P:L ≥ 1:1000 at pH 7. The activity of MelP5 decreases slightly at pH 4 for unknown reasons but it still shows high leakage at P:L ≥ 1:500. In contrast, at pH 7, MelP5_Δ4 and MelP5_Δ6 are essentially inactive, even at P:L = 1:100, consistent with the fact that they do not bind well and are not helical at pH 7. At pH 4, MelP5_Δ4 and MelP5_Δ6 are membrane active, causing significant release of ANTS/DPX at 1:100. However their potency at pH 4 is somewhat less than the potency of MelP5, causing less than 20% release at P:L = 1:500.
Figure 4.
Small solute leakage. Leakage of ANTS and DPX from POPC vesicles was measured after addition of the peptides MelP5, MelP5_Δ4 and MelP5_Δ6. In each case, 1 mM POPC vesicles containing entrapped ANTS and DPX were used. Leakage was determined from the increase in ANTS fluorescence after peptide addition, relative to the increase in ANTS fluorescence after addition of the detergent Triton X-100, at the end of the measurement. Each left panel shows kinetic traces for leakage at pH 4 at P:L ratios of 1:100, 1:500 and 1:1000. At 200 seconds, Triton X-100 was added to cause complete release. The three right panels show the fractional leakage caused by the peptides at the three P:L ratios, and at pH 4 and pH 7, just before Triton addition.
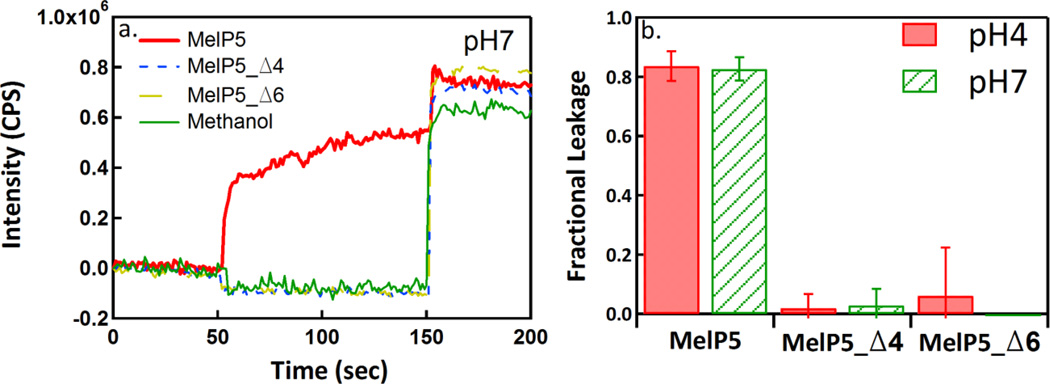
Lastly, we assessed the ability of the new peptides to release macromolecules from lipid vesicles. MelP5, as reported elsewhere (12), is unique among pore forming peptides in that it forms pores that enable passage of macromolecules even at low P:L ratios (P:L ≤ 1:100). Here we measured the release of a 10,000 Da dextran at P:L = 1:100 at pH 4 and pH 7 using a FRET-based assay described in Materials and Methods. In Figure 5, we show that MelP5 releases nearly 100% of the dextran molecules at both pH 4 and pH 7 when P:L = 1:100. Under the same conditions, neither MelP5_Δ4 nor MelP5_Δ6 cause any measurable dextran release at either pH, despite the fact that they release small molecules very efficiently at pH 4 and P:L = 1:100.
Figure 5.
Macromolecular leakage. Panel a shows example FRET measurements of leakage caused by MelP5, MelP5_Δ4 and MelP5_Δ6. Lipid concentration was 1 mM and P:L was 1:100. Excitation of Alexafluor488-streptavidin was at 495 nm and emission of biotin-dextran(10kD)-TAMRA was at 588 nm. An increase in FRET indicates dextran leakage and complex formation. At 150 seconds, Triton X-100 was added to completely permeabilize the vesicles. Panel b shows the fractional leakage of dextran at P:L = 1:100 for all three peptides at both pH 4 and pH 7.
DISCUSSION
Here, we sought to engineer membrane active peptides with new activities by combining the properties of several well-understood peptides. Specifically, we combined the properties of a unique macromolecular-sized pore-forming peptide, MelP5, with those of two pH sensitive, membrane active peptides, GALA and pHLIP. We hypothesized that by replacing 4 or 6 carefully selected residues, out of 26, with glutamate or aspartate we could gain the ability to control peptide activity, such that activity would occur only at acidic pH. Fluorescence based binding assays and CD measurements demonstrated that the analogs that we created do have pH sensitive membrane binding and secondary structure, with apparent pKa values between 4.6 and 5.0. The two analogs also caused bilayer permeabilization at low pH, but were inactive ay neutral pH. However, they did not possess the unique ability of MelP5 to cause macromolecule-sized pores in membranes.
Thus, this work shows that the properties of MelP5, pHLIP, and GALA are additive, but only partially so. Previously, it has been suggested that the limitations in peptide engineering come from the lack of knowledge of the molecular mechanism of action of membrane active peptides (10, 27). Here we demonstrate an additional roadblock: the lack of additivity of membrane active properties, even for very well characterized peptides.
Based on our findings, we propose that an approach based on combinatorial chemistry and high-throughput screening (11, 28) may be the most efficient way to engineer membrane active peptides with specific properties. This approach requires that a peptide library is designed and then screened using high throughput assays. The approach has already yielded new peptides that can form large equilibrium pores (both α-helical and β-sheet) (11), peptides that translocate spontaneously across bilayers (29), and antimicrobial peptides (28). As the assays used here can be run in a high throughput format, we are hopeful that peptides will eventually be identified that are triggered by low pH to form macromolecular-sized pores in lipid bilayers.
Highlights.
The goal was to design pH-sensitive peptides that form large pores in bilayers
Sequences causing macromolecular poration and pH-sensitive insertion were combined
The engineered peptides had pH-sensitive binding, structure and permeabilization
Addition of pH-sensitivity abolished macromolecule-sized permeabilization
Lack of additivity is a roadblock to the engineering of membrane active peptide
ACKNOWLEDGEMENTS
Supported in part by NIH GM095930 to KH, NSF DMR 1003411 to WCW, and NSF MCB 1157687 to KH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
William C. Wimley, Email: wwimley@tulane.edu.
Kalina Hristova, Email: kh@jhu.edu.
Reference List
- 1.Shai Y. Mode of action of membrane active antimicrobial peptides. Biopolymers. 2002;66:236–248. doi: 10.1002/bip.10260. [DOI] [PubMed] [Google Scholar]
- 2.Hancock REW, Lehrer R. Cationic peptides: A new source of antibiotics. Trends Biotech. 1998;16:82–88. doi: 10.1016/s0167-7799(97)01156-6. [DOI] [PubMed] [Google Scholar]
- 3.Brown KL, Hancock REW. Cationic host defense (antimicrobial) peptides. Current Opinion in Immunology. 2006;18:24–30. doi: 10.1016/j.coi.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Easton DM, Nijnik A, Mayer ML, Hancock REW. Potential of immunomodulatory host defense peptides as novel anti-infectives. Trends Biotech. 2009;27:582–590. doi: 10.1016/j.tibtech.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hancock REW, Sahl HG. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nature Biotechnology. 2006;24:1551–1557. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- 6.Jenssen H, Hamill P, Hancock REW. Peptide antimicrobial agents. Clinical Microbiology Reviews. 2006;19:491. doi: 10.1128/CMR.00056-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marcos JF, Gandia M. Antimicrobial peptides: to membranes and beyond. Expert Opinion on Drug Discovery. 2009;4:659–671. doi: 10.1517/17460440902992888. [DOI] [PubMed] [Google Scholar]
- 8.Nicolas P. Multifunctional host defense peptides: intracellular-targeting antimicrobial peptides. Febs Journal. 2009;276:6483–6496. doi: 10.1111/j.1742-4658.2009.07359.x. [DOI] [PubMed] [Google Scholar]
- 9.Wimley WC. Describing the mechanism of antimicrobial peptide action with the interfacial activity model. ACS Chem. Biol. 2010;5:905–917. doi: 10.1021/cb1001558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wimley WC, Hristova K. Antimicrobial Peptides: Successes, Challenges and Unanswered Questions. J. Membr. Biol. 2011;239:27–34. doi: 10.1007/s00232-011-9343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krauson AJ, He J, Wimley WC. Gain-of-Function Analogues of the Pore-Forming Peptide Melittin Selected by Orthogonal High-Throughput Screening. J. Am. Chem. Soc. 2012;134:12732–12741. doi: 10.1021/ja3042004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiedman G, Fuselier T, He J, Searson PC, Hristova K, Wimley WC. Highly Efficient Macromolecule-Sized Poration of Lipid Bilayers by a Synthetically Evolved Peptide. J. Am. Chem. Soc. 2014;136:4724–4731. doi: 10.1021/ja500462s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiedman G, Herman K, Searson P, Wimley WC, Hristova K. The electrical response of bilayers to the bee venom toxin melittin: Evidence for transient bilayer permeabilization. Biochim. Biophys. Acta. 2013;1828:1357–1364. doi: 10.1016/j.bbamem.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shai Y, Oren Z. From "carpet" mechanism to de-novo designed diastereomeric cell-selective antimicrobial peptides. Peptides. 2001;22:1629–1641. doi: 10.1016/s0196-9781(01)00498-3. [DOI] [PubMed] [Google Scholar]
- 15.Yao L, Daniels J, Wijesinghe D, Andreev OA, Reshetnyak YK. pHLIP (R)-mediated delivery of PEGylated liposomes to cancer cells. J. Control. Release. 2013;167:228–237. doi: 10.1016/j.jconrel.2013.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andreev OA, Engelman DM, Reshetnyak YK. pH-sensitive membrane peptides (pHLIPs) as a novel class of delivery agents. Molecular Membrane Biology. 2010;27:341–352. doi: 10.3109/09687688.2010.509285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fendos J, Barrera FN, Engelman DM. Aspartate Embedding Depth Affects pHLIP's Insertion pK(a) Biochemistry. 2013;52:4595–4604. doi: 10.1021/bi400252k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parente RA, Nir S, Szoka FC., Jr Mechanism of leakage of phospholipid vesicle contents induced by the peptide GALA. Biochemistry. 1990;29:8720–8728. doi: 10.1021/bi00489a031. [DOI] [PubMed] [Google Scholar]
- 19.Subbarao NK, Parente RA, Szoka FC, Nadasdi L, Pongracz K. pH-dependent bilayer destabilization by an amphipathic peptide. Biochemistry. 1987;26:2964–2972. doi: 10.1021/bi00385a002. [DOI] [PubMed] [Google Scholar]
- 20.Mayer LD, Hope MJ, Cullis PR. Vesicles of variable sizes produced by a rapid extrusion procedure. Biochim. Biophys. Acta. 1986;858:161–168. doi: 10.1016/0005-2736(86)90302-0. [DOI] [PubMed] [Google Scholar]
- 21.Hope MJ, Bally MB, Mayer LD, Janoff AS, Cullis PR. Generation of multilamellar and unilamellar phospholipid vesicles. Chem. Phys. Lipids. 1986;40:89–107. [Google Scholar]
- 22.Stewart JC. Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Anal. Biochem. 1980;104:10–14. doi: 10.1016/0003-2697(80)90269-9. [DOI] [PubMed] [Google Scholar]
- 23.Ladokhin AS, Jayasinghe S, White SH. How to measure and analyze tryptophan fluorescence in membranes properly, and why bother? Anal. Biochem. 2000;285:235–245. doi: 10.1006/abio.2000.4773. [DOI] [PubMed] [Google Scholar]
- 24.White SH, Wimley WC. Membrane protein folding and stability: Physical principles. Annu. Rev. Biophys. Biomol. Struc. 1999;28:319–365. doi: 10.1146/annurev.biophys.28.1.319. [DOI] [PubMed] [Google Scholar]
- 25.Wimley WC, White SH. Experimentally determined hydrophobicity scale for proteins at membrane interfaces. Nature Struct. Biol. 1996;3:842–848. doi: 10.1038/nsb1096-842. [DOI] [PubMed] [Google Scholar]
- 26.White SH, Wimley WC, Ladokhin AS, Hristova K. Protein folding in membranes: Determining the energetics of peptide-bilayer interactions. Methods Enzymol. 1998;295:62–87. doi: 10.1016/s0076-6879(98)95035-2. [DOI] [PubMed] [Google Scholar]
- 27.Almeida PF, Pokorny A. Mechanisms of antimicrobial, cytolytic, and cell-penetrating peptides: from kinetics to thermodynamics. Biochemistry. 2009;48:8083–8093. doi: 10.1021/bi900914g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rathinakumar R, Walkenhorst WF, Wimley WC. Broad-Spectrum Antimicrobial Peptides by Rational Combinatorial Design and High-Throughput Screening: The Importance of Interfacial Activity. J. Am. Chem. Soc. 2009;131:7609–7617. doi: 10.1021/ja8093247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marks JR, Placone J, Hristova K, Wimley WC. Spontaneous membrane-translocating peptides by orthogonal high-throughput screening. J. Am. Chem. Soc. 2011;133:8995–9004. doi: 10.1021/ja2017416. [DOI] [PMC free article] [PubMed] [Google Scholar]