Abstract
Replacing mouse Cyp1a with human CYP1A enables the humanized CYP1A mice to mimics human metabolism of the dietary carcinogen, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), by N2-hydroxylation to a proximate carcinogen. Our previous study demonstrated that PhIP, in combined with the dextrin sulfate sodium (DSS)-induced colitis, induces colon carcinogenesis in hCYP1A mice. Here, we employed whole exome sequencing and found multiple gene mutations in PhIP/DSS-induced colon tumors. Mutations in the exon 3 of Ctnnb1/β-catenin, however, were the predominant events. We further sequenced the key fragments of Apc, Ctnnb1, and Kras, because mutations of these genes in the humans are commonly found as the drivers of colorectal cancer. Mutations on either codon 32 or 34 in the exon 3 of Ctnnb1 were found in 39 out of 42 tumors, but no mutation was found in either Apc or Kras. The sequence context of codons 32 and 34 suggests that PhIP targets +3G in a TGGA motif of Ctnnb1. Since mutations that activate Wnt signal is a major driving force for human colorectal cancers, we conclude that the mutated β-catenin is the driver in PhIP/DSS-induced colon carcinogenesis. This result suggests that the colon tumors in hCYP1A mice mimic human colorectal carcinogenesis not only in the dietary etiology involving PhIP, but also in the aberrant activation of the Wnt signaling pathway as the driving force.
Keywords: Colorectal cancer, carcinogenesis, exon sequencing, driver mutation
Introduction
Colorectal cancer (CRC) is among the top three cancers in both women and men that cause morbidity and mortality. In the US, about 150,000 new cases are diagnosed every year. Even though the survival rate for CRC has improved during the last decade, about one third of patients die from this disease, which accounts for about 10% of cancer associated death [1]. The conversion of normal intestinal epithelial cells to microadenoma and adenocarcinoma, and further progression to metastatic cancer involves a series of activation of oncogenes and inactivation of tumor suppressors [2]. The prominent genetic events include mutations in key components of the Wnt signaling (e.g. APC), the proliferation signaling (e.g. PI3K, AKT, K-ras), the TGFβ signaling, and DNA damage response and repair (e.g. p53, mismatch repair MMR genes MMRs) [2]. About 90% human CRCs are found harboring dominant active mutations in the Wnt signaling pathway components; among them, mutations in APC gene account for the majority (~70-80%) [2]. An alternative to APC mutation is the active mutation of β-catenin/CTNNB1 gene, which is found in ≤5% human CRCs but at a much higher frequency in hereditary nonpolyposis colorectal cancer (HNPCC) [3]. Mutations in other components (e.g. AXIN1, AXIN2, Dickkopf-1) that lead to the activation of Wnt signaling are also reported [2]. Recent study on human CRC using the next generation sequencing technology further documents the importance of these mutations in human CRC [4]. Epigenetic alterations are also demonstrated to play important roles in colorectal carcinogenesis [5]. These alterations, especially the genetic mutations, are recognized for their roles in the different stages of CRC development [6]. For instance, APC plays the gatekeeper role in normal colon epithelial cells and the loss of APC results in the activation of Wnt signaling and the subsequent imbalance of cell proliferation over cell death that leads to the earlier lesion such as microadenoma [7]. Overall, the findings of these disease-associated genetic/epigenetic events reveal molecular mechanism of colorectal carcinogenesis, and these events provide potential biomarkers for diagnosis and prognoses as well as potential targets for therapy.
It is well recognized that the risk of cancer is associated with life style and environment factors [8-10]. Diet and nutrition are estimated to be related to up to 50% of human CRC worldwide [10]. Some dietary factors are known to have carcinogenic activity. One group of such chemical is heterocyclic amines (HCAs), which are found in cooked meat and fish [11,12]. HCAs are listed in the US Department of Health and Human Services (2005) as chemical “reasonably anticipated to be a human carcinogen”. Human exposure to HCAs is in a wide range (0.1-12 μg/day) [13]. PhIP is the most common and the most abundant HCAs [13,14]. Daily intake of PhIP by Americans is estimated to be 280-460 ng/day per person [15].
HCAs are metabolically activated in vivo to proximate and ultimate carcinogens. In human, PhIP as well as other HCAs is mainly metabolized through N-hydroxylation by cytochrome P450 (CYP) 1A2 and then conjugated by N-acetyltransferase or sulfotransferase [12,16,17]. The acetoxy or sulfate metabolite is spontaneously converted to arylnitrenium ion (R-NH+), which can react with DNA to form adduct at the 8-position carbon of deoxyguanine base, dG-C8-PhIP. In human colon samples, dG-C8-PhIP is detected at approximately 3 adducts per 108 nucleotides or about 20 adducts per cells [18]. Indeed, a fast acetylator polymorphic trait of N-acetyltransferase NAT2 (i.e. NAT2*4) has been associated with an increased risk of CRC [19]. PhIP-DNA adduct was also reported in human prostate [20]. In the experimental studies using rodents, PhIP-DNA adduct has been found in different organs (e.g. liver, colon, prostate, lung) [12,13,17,21,22]. In mouse, PhIP is mainly metabolized by Cyp1a2 through 4’-hydroxylation, which leads to detoxification [16], and a higher dose of PhIP is required to induced tumor and the induction is independent of the activation of PhIP by Cyp1a2 [23]. Thus, mouse is not a suitable experimental model to mimic PhIP-induced carcinogenesis in humans. To overcome this limitation, we have used the hCYP1A mice, in which the mouse Cyp1A gene was replaced by human CYP1A gene [24,25] and demonstrated that PhIP induces colon and prostate carcinogenesis, recapuliating several key features of human cancers [26,27]. Given the fact that high temperature cooked meat and fish are very common in the western diet, the PhIP-induced colon cancer in hCYP1A mice would share the dietary etiology of certain types of human CRC.
In this study, we used exon-sequencing technology and selected fragments for Sanger sequencing to analyze the genetic alterations in the exome of the PhIP-induced colon tumors. Our result demonstrated β-catenin/Ctnnb1 gene as the predominant target of PhIP, and the observed mutations of this gene are expected to activate the Wnt signaling pathway, which drives colon carcinogenesis.
Materials and Methods
Chemicals
PhIP-HCl was purchased from Wake Chemicals USA, Inc. (Richmond, VA) and dissolved in milliQ water. Dextran sulfate sodium (DSS; molecular weight 35,000 – 44,000) was purchased from MP Biomedicals (Solon, OH) and dissolved in milliQ water to 1.2% (w/v).
Animals and procedures
Humanized CYP1A mice (hCYP1A), carrying human CYP1A gene to replace mouse Cyp1A gene, were generated as described previously [26]. All animal procedures were in accordance with the animal study protocol (No. 02-027) approved by the Rutgers University Institutional Animal Care and Use Committee. Mice were maintained in a sterile room of animal facility under standard 12 hour light/12 hour dark cycle with water and diet provided ad libitum unless otherwise specified. At the age of Week 5, mice were switched from a normal chew diet to the AIN93M diet (Research Diets, New Brunswick, NJ) throughout the experimental study and body weights were measured weekly. At Week 6, the mice was treated with a dose of PhIP (200 mg/kg body weight) by oral gavage. After 1 week, the mice were administered 1.5% (w/v) DSS in drinking water for a week. During this time period, mice were monitored daily for changes in body weight and rectal bleeding. Mice were then returned to regular drinking water and continuously fed on AIN93M diet without any other treatment until sacrifice at 10 weeks after the PhIP treatment. After mice were sacrificed by CO2 asphyxiation, the colon was excised, flushed with saline, the length measured, cut longitudinally, and flattened on filter paper. The number, size and location of visible tumors were recorded under dissecting microscope. The tumors were dissected under dissecting microscope and stored in RNAlater solution (Qiagen, Valencia, CA) at −80°C. After the tumors were removed, the mucosal epithelium layer in the descending colon were separated from the muscle layer by a plastic scratcher and stored in RNAlater solution to serve as the normal matching control.
DNA/RNA extraction and exonome sequencing
To extract total RNA and genomic DNA from colon tumors and its matching normal colon tissue, the tissues stored in RNAlater were homogenized in the lysis buffer of AllPrep DNA/RNA Mini Kit (Qiagen) using Omni Bead Raptor 24 (Omni International, Kennesaw, GA). DNA and RNA were then purified according to the manufacturer protocol. The quality of DNA and RNA were examined by gel electrophoresis.
For genetic analysis, the medium size tumors from two mice were used: two tumors and one matching normal control samples for each mouse. Exome sequencing of these six samples was performed using Aglient Mouse Exome Sequencing Kit on the Applied Biosystems SOLiD system done by ShanghaiBio Co. at USA (North Brunswick, NJ). The captured sequence was then analyzed by Extensible Genomics Toolbox of DNAnexsus (Mountain View, CA).
PCR and DNA Sanger sequencing
The N-terminal half (6kb) of the coding sequence of Apc was amplified by RT-PCR in 5 1.2-1.5kb overlapping fragment using RNA samples with SuperScript First-Strand reverse transcription kit (Life Technologies, Grand Island, NY) and Advantage 2 PCR kit (Clontech, Mountain View, CA). Apc fragment (equal to position 3600bp to 5600bp in coding region) containing mutation cluster region (MCR), Ctnnb1 exon 3, and Kras exon 1 were directly amplified using genomic DNAs with Advantage 2 PCR kit (Clontech). The sequence information of all primers is listed in Table 1. The amplified products were separated by electrophoresis and then purified using the QIAquick Gel Extraction Kit (Qiagen). The purified products were sequenced using Sanger sequence method from both directions with PCR primers by Genewiz Inc. (South Plainfield, NJ).
Table 1.
Sequence information of primers used in PCR to characterize Apc, Ctnnb1, and Kras.
| Gene and Target Region | PCR Primers | Product Size (bp) | |
|---|---|---|---|
| Apc | |||
| mRNA −30 to 1333 | Forward | GTCTGGCAGGTCCAAGGGTA | 1356 |
| Reverse | CTGATGCTCAACAGGAGCTG | ||
| mRNA 1200 to 2600 | Forward | TCCGAGTCCTTCATCTTTTGGAAC | 1470 |
| Reverse | TTTCTGTTGTTGGATGGTAAGCACTGAGGC | ||
| mRNA 2450 to 3900 | Forward | TGACTGTTCTTTCACCATATTTAAATACTA | 1338 |
| Reverse | CTTCTGCTGTCTGCAGAGTATTAG | ||
| mRNA 3600 to 5000 * | Forward | CATCAGCACAAAGCACTAAACCTG | 1474 |
| Reverse | CCAATTCATTTGGAGGGGACTCTA | ||
| mRNA 4850 to 6135 | Forward | CAGCACAGAATAGGCTGCAGGCAC | 1568 |
| Reverse | GGCAGAACTTATACACTCCTGTAACA | ||
| Ctnnb1 | |||
| Partial intron 2 - exon 3 | CTAACATACTCTGTTTTTACAGCTGAC | 250 | |
| CAGCTACTTGCTCTTGCGTGA | |||
| Kras | |||
| Exon 1 (G12 region) | GCCTGCTGAAAATGACTGAG | 116 | |
| ATCGTAGGGTCATACTCATCCAC | |||
Since this fragment contains MCR (~3750-4500 bp), this set of primers were also used to amplify genomic DNA for Sanger sequence.
Results
1. Somatic genetic signature of PhIP/DSS-induced colon tumor as determined by exome sequencing
We sequenced the exome of 4 PhIP-induced colon tumors and 2 corresponding nontumorous colon tissue samples as controls. These samples were from two randomly selected male mice: one mouse had 4 tumors and the other had 7 tumors. We took two tumors and 1 control from each mouse. The carcinogenesis experiment consisted of 10 mice treated with PhIP and DSS, and the average tumor number in this experiment was about 4, which was in the normal range [26]. The tumors at this stage were tubular adenocarcinoma, showing tubular architecture featured with the surface epithelial dysplasia that extends downward in the base, serrated crypt lumens with stratified pencil-like nuclei in some crypts and nuclear atypia in the majority of the tumor cells, as well as some microinvasive features, as described in our previous report [26]. We completely sequenced 4.3-6.3×109 bases, and over 90% of reads were mapped to the mm9 mouse (Mus musculus) genome (http://genome.ucsc.edu) for all samples (Table 2). These results covered completely over 98.2% exons; for each sample, only about 1.2% exons were partial covered, and only less than 0.2% exons were not covered (Table 2). The 14 exons in Cyp1a (Cyp1a1 and 1a2) gene, which were knocked out in this transgenic mouse, were completely not detected in all 6 samples, whereas the exons of adjacent genes were well fully covered (Supplemental Fig. 1). This data supports the high confidence of this exome sequencing result.
Table 2.
Whole-Exome sequencing Summary
| Mouse No.1 | Mouse No. 2 | |||||
|---|---|---|---|---|---|---|
| Control | Tumor 1 | Tumor 2 | Control | Tumor 1 | Tumor 2 | |
| Total bases | 4,356,463,400 | 4,814,310,600 | 6,367,888,800 | 4,816,666,800 | 4,973,358,000 | 6,003,544,400 |
| Total Read | 43,564,634 | 48,143,106 | 63,678,888 | 48,166,668 | 49,733,580 | 60,035,444 |
| MAPed Read | 40,706,535 | 45,059,045 | 58,463,734 | 44,731,090 | 46,600,323 | 55,529,103 |
| % of MAPed | 93% | 93.59% | 91.81% | 92.87% | 93.70% | 92.49% |
| Exon Coverage | ||||||
| Completely | 204,240 | 204,863 | 204,990 | 204,665 | 205,045 | 204,978 |
| Partial | 3,117 | 2,625 | 2,560 | 2,811 | 2,482 | 2,587 |
| Unsequenced | 443 | 312 | 250 | 324 | 273 | 235 |
By examining the tumor-normal pairs, we identified 1110 to 1482 somatic mutations, including nucleotide substitutions, insertions, and deletions in the exome (Table 3). Among them, over 80% were single nucleotide substitutions. The majority of mutations were heterozygous. There were more mutations on G/C than on A/T. The mutation rates in the exon area in tumors were 20-27 per 106 bases. According to the exome data of human CRCs [4], tumors bearing mutation rates over 8.2 per 106 bases are considered as hypermutated; thus, PhIP/DSS-induced tumors are hypermutated tumors. In terms of protein coding, these mutations included 695-885 non-synonymous mutations and 23-31 reading frame shifts; a few of them are expected to be cancer-drivers, while the other are the passenger mutations.
Table 3.
Genetic mutations in the whole-exome of PhIP-induced colon tumors
| Mouse 1 | Mouse 2 | |||
|---|---|---|---|---|
| Tumor 1 | Tumor 2 | Tumor 1 | Tumor 2 | |
| In coding DNA sequence | 1110 | 1198 | 1321 | 1482 |
| Single nucleotide substitution | 927 | 991 | 1155 | 1276 |
| Multiple nucleotide substitution | 126 | 158 | 121 | 144 |
| Insertion | 29 | 23 | 26 | 31 |
| Deletion | 28 | 26 | 19 | 31 |
| Deletion in GC | 23 | 23 | 13 | 28 |
| Mixed | 0 | 0 | 0 | 0 |
| Heterozygous | 963 | 1059 | 928 | 1046 |
| Mutation in A or T | 428 | 492 | 549 | 607 |
| Mutation in G or C | 702 | 752 | 800 | 906 |
| In Codon | ||||
| Synonymous | 357 | 412 | 517 | 534 |
| Non-Synonymous | 695 | 722 | 748 | 885 |
| frameshift | 23 | 30 | 24 | 31 |
2. Common mutations in PhIP/DSS-induced tumors
We identified a group of 43 genes carrying non-synonymous mutations or reading frame shift in all four tumors (Supplemental Table 1). Most of these genes carry multiple mutations in each tumors. In total, these 43 genes carried 120 non-synonymous mutations or reading frame shift in each tumors. Since it has been suggested that cancer driver gene does not need more than one alteration to initiate/promote cancer [28], it is likely that most of these genes were mutated by PhIP but not dirvers or mutated at the later stages of carcinogenesis. There were 5 genes (Fam129a, Fcnb, V1rc1, Zbtb2, and Dusp14) in which only one mutation was observed in each tumor. According to the literature, however, Zbtb2 and Dusp14 have only minimum involvement in signaling regulation, and no involvement in carcinogenesis. Mutation frequencies of these genes in human CRCs are also extremely low [4]. Therefore, it is less likely that these mutations play significant roles in PhIP/DSS-induced colon carcinogenesis. We next reduced the searching creatic to the genes having mutations in “at least in three tumors”, and identified additional 43 genes carrying non-synonymous mutations or reading frame shift in at least three tumors (Table 4). Within this group, there were 26 genes that carry no more than one mutation in each tumor. Among these genes, Ctnnb1, Hoxa10, and Maml3 are potential candidates as the driver genes. Mutant Ctnnb1 is a well-documented cancer driver, especially in CRC [2]. Deregulated expression of Hoxa10 has been reported in gastric cancer [29]. Maml3 is essential for Notch signaling [30], which plays critical role in the development of human CRC. No literature supports the role of other genes involved in carcinogenesis.
Table 4.
Genes carrying non-synomnous mutation in three tumors.
| Chromosome No. | Gene | Numbers of Variations | |||
|---|---|---|---|---|---|
| Mouse 1 | Mouse 2 | ||||
| Tumor 1 | Tumor 2 | Tumor 1 | Tumor 2 | ||
| chrl | Rrsl | l | 6 | 4 | |
| chrl | Ptprv | l | 4 | 4 | |
| chr2 | Crb2 | l | l | l | |
| chr2 | Fmnl | l | l | l | |
| chr2 | Sirpa | 3 | 2 | 3 | |
| chr2 | Nsfllc | 4 | 2 | l | |
| chr2 | Olfr1261 | l | 2 | 3 | |
| chr2 | Mageb3 | 2 | l | l | |
| chr3 | Gm5l50 | l | l | l | |
| chr3 | Medl2l | l | l | l | |
| chr3 | Maml3 | l | l | l | |
| chr4 | Ftl2 | l | 3 | 2 | |
| chr5 | Vmn2rl4 | l | l | l | |
| chr5 | Speer4b | l | l | l | |
| chr6 | Olfr2l3 | l | 3 | l | |
| chr6 | HoxalO | l | l | l | |
| chr6 | Zfml | l | l | l | |
| chr6 | Ppp4r2 | l | 2 | 2 | |
| chr6 | Prp2 | l | 5 | 3 | |
| chr7 | Hbb-bl | l | 2 | l | |
| chr7 | Hbb-b2 | l | 2 | l | |
| chr7 | Lrrc68 | l | l | l | |
| chr8 | Csmdl | l | l | l | |
| chr8 | Thapl | l | l | l | |
| chr8 | Cdhll | l | l | l | |
| chr8 | Cdtl | 2 | l | l | |
| chr9 | Ddx6 | 2 | l | l | |
| chr9 | Crtap | l | l | l | |
| chr9 | Mfrp | l | l | l | |
| chr9 | Ctnnbl | l | l | l | |
| chr9 | Apoa4 | 4 | 3 | l | |
| chr9 | Atr | 2 | l | l | |
| chrli | Nlrpla | 2 | l | 3 | |
| chrli | Olfr463 | l | l | l | |
| chrli | Elac2 | l | l | l | |
| chrli | Cogl | l | l | l | |
| chr12 | Syne2 | l | l | l | |
| chr13 | Heatrl | l | l | l | |
| chr13 | Nlrp4f | 2 | l | 3 | |
| chr14 | Vmn2r89 | 4 | 1 | 1 | |
| chr14 | Mycbp2 | 1 | 1 | 1 | |
| chr15 | Larp4 | 1 | 1 | 1 | |
| chr15 | Krt81 | 1 | 1 | 1 | |
| chr16 | Cd200r2 | 4 | 2 | 1 | |
| chr17 | Vmn2r115 | 3 | 3 | 2 | |
| chr17 | Slc22a7 | 1 | 1 | 1 | |
| chr19 | Cyp2c39 | 1 | 2 | 1 | |
| chr19 | Cyp2c50 | 1 | 1 | 1 | |
| chrX | Las1l | 2 | 1 | 2 | |
| chrX | Diap2 | 1 | 2 | 1 | |
| Total (Genes) | 43 | ||||
| Total (Mutations) | 44 | 42 | 48 | 41 | |
We then focused on the location of mutations. We found 29 mutations in the coding regions of 19 genes that lead to non-synonymous substitution or reading frame shift in all four tumors (Supplemental Table 2). As expected, these common mutations were among the genes identified in Supplemental Table 1; but they localized in the genes carrying multiple mutations, suggesting that these mutations are not the drivers. Then, we again reduced the searching critic to the genes having mutations in “at least in three tumors”, and identified 61 mutations in the coding regions of 41 genes (Supplemental Table 3). The majority of mutations in this list were in the genes that were identified in Supplemental Table 1 and Table 4. The only mutation with known impact in carcinogenesis is a common mutation in Maml3 found in three tumors. However, this mutation was a deletion of a CAG from a stretch of ~70 CAG repeats, which is unlikely to significantly impact the function of Maml3 protein. Thus, to narrow the searching list by mutation position did not provide more insightful information. These common mutations may represent the fragile sites in mouse genome when mice were treated in our experimental conditions.
Nevertheless, the above analyses on the exomes of 4 tumors reveal that 3 out of 4 tumors carry somatic mutations in Ctnnb1, of which the role is well recognized in CRC. The mutations were on codon 32 in tumor 1 and 3 and codon 34 in tumor 4. The amino acid residues encoded by codon 32 and 34 are the sites mediating the recognition and binding of ubiquitin ligase; mutations on codons 32 and 34 display stronger transformation activity than other mutations, such as codon 33 [31,32]. These mutations have been reported in human CRC [2,33,34]. Thus, somatic mutation in Ctnnb1 is the candidate for the driver event in PhIP/DSS-induced colon carcinogenesis in hCYP1A mice.
3. Fragment sequencing reveals the dominant active mutations in Ctnnb1 in majority of PhIP/DSS-induced tumors
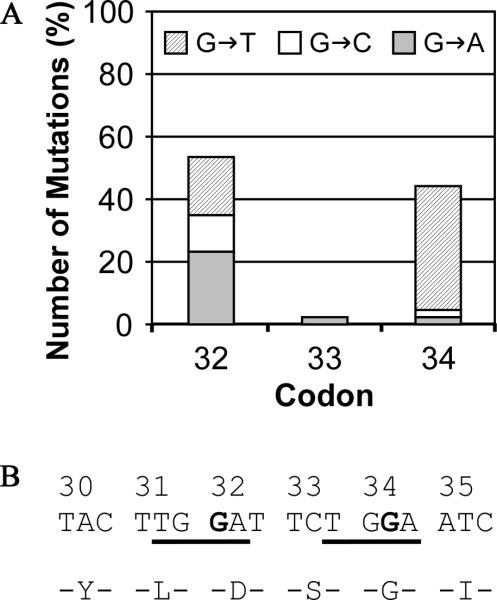
Above, we identified the dominant activation mutations in Ctnnb1 in 3 out 4 PhIP/DSS-induced tumors. However, we did not found any alteration in other well-known CRC genes such as APC and Kras. To validate this result in more tumors, we next selected 11 mice (6 males and 5 females) from two experiments, dissected all visible tumors (a total of 42, including the 4 samples used for exome sequencing) and the adjacent normal control epithelial tissues. The DNA samples were used for Sanger-sequencing to examine the key regions of Ctnnb1, Apc, and Kras genes, of which human counterparts are CRC drivers. We focused on the N-terminal half of the coding sequence of Apc, because human APC gene mutations are predominately in the N-terminal half and the majority of somatic mutations locate in the so-called mutation cluster region (MCR) within N-terminal half [2,33,35]. For Ctnnb1, we focused on Exon 3, encoding the motif consisting of phosphorylation and ubiquitin ligase recognition sites, in which mutations cause dominant active Wnt signaling and are frequently found in human CRC [2,33,34]. For Kras, we focused on exon 1 containing codon 12, of which the mutation is a well-characterized oncogenic event in a wide range of human cancers, including CRC [2]. The sequencing results in 42 tumors plus 11 matching controls showed that there was no mutation in Apc and Kras, but there were high frequent mutations on Ctnnb1 codons 32 and 34. In 42 tumors, we found mutations on Ctnnb1 in 39 tumors, no mutation in 2 tumors and inconclusive for 1 tumor (Table 5). Among the 39 mutant Ctnnb1 tumors, there were 36 tumors carrying only one mutation, on either codon 32 and 34; while 2 tumors carrying both codon 32 and 34 mutations. All mutations on codons 32 and 34 are on G. On codon 32, there was no preference in the conversion of G to A/C/T resulting in the substitution of Asp by Asn, Tyr or His; whereas, on codon 34, the majority of mutation was the conversion of G to T, resulting in the substitution of Gly to Val (Fig. 1A). Presumably, cells carrying the oncogenic events resulted from these substitutions acquired growth advantage. The context sequences in codon 32 and 34 imply that it is the +3 G substitution in a TGGA motif (Fig. 1B). Overall, oncogenic mutations on Ctnnb1 were found in the majority (>92%) of PhIP/DSS induced colon tumors in hCYP1A mice.
Table 5.
Mutations in Ctnnb1 codons 32, 33, and 34 in PhIP-induced hCYP1A mouse colon tumors.
| Mouse No. and Sex | DNA ID | Codon 32 | Codon 33 | Codon 34 | |||
|---|---|---|---|---|---|---|---|
| Mutation* | AA change | Mutation | AA change | Mutation | AA change | ||
| 1 (M) | Control†,‡ | wt | |||||
| Tumor 1 | GGA→GTA | Gly→Val | |||||
| Tumor 2‡ | GAT→CAT | Asp→His | |||||
| Gly→Leu | |||||||
| Tumor 3‡ | GGA→TTA§ | /stop/Val§ | |||||
| Tumor 4 | TCT→TAT | Ser→Tyr | |||||
| 2 (M) | Control‡ | wt | |||||
| Tumor 1 | GGA→GTA | Gly→Val | |||||
| Tumor 2 | GGA→GTA | Gly→Val | |||||
| Tumor 3‡ | GAT→AAT | Asp→Asn | |||||
| Tumor 4 | GAT→TAT | Asp→Tyr | |||||
| Tumor 5 | GAT→TAT | Asp→Tyr | |||||
| Tumor 6 | GAT→AAT | Asp→Asn | |||||
| Tumor 7‡ | GGA→GTA | Gly→Val | |||||
| 3 (M) | Control | wt | |||||
| Tumor 1 | GGA→GTA | Gly→Val | |||||
| Tumor 2 | GAT→AAT | Asp→Asn | |||||
| Tumor 3 | GGA→GTA | Gly→Val | |||||
| 4 (M) | Control | wt | |||||
| Tumor 1 | GAT→TAT | Asp→Tyr | |||||
| Tumor 2 | GAT→TAT | Asp→Tyr | |||||
| 5 (M) | Control | wt | |||||
| Tumor 1 | GGA→GTA | Gly-Val | |||||
| Tumor 2 | GAT→TAT | Asp→Tyr | |||||
| Tumor 3 | GAT→TAT | Asp→Tyr | |||||
| Tumor 4 | GGA→CGA | Gly→Arg | |||||
| 6 (M) | Control | wt | |||||
| Tumor 1 | GGA→GTA | Gly→Val | |||||
| Tumor 2 | wt | ||||||
| Tumor S | GAT→AAT | Asp→Asn | |||||
| 7 (F) | Control | wt | |||||
| Tumor 1 | GGA→GTA | Gly→Val | |||||
| Tumor 2 | GAT→T/AAT | Asp→Tyr/Asn | |||||
| Tumor 3 | GAT→AAT | Asp→Asn | |||||
| Tumor 4 | GAT→AAT | Asp→Asn | GGA→GTA | Gly→Val | |||
| Tumor 5 | GGA→GT/AA | Gly→Val/Glu | |||||
| 8 (F) | Control | wt | |||||
| Tumor 1 | GAT→TAT | Asp→Tyr | |||||
| 9 (F) | Control | wt | |||||
| Tumor 1 | GGA→GTA | Gly→Val | |||||
| Tumor 2 | GAT→AAT | Asp→Asn | |||||
| Tumor 3 | GAT→CAT | Asp→His | |||||
| Tumor 4 | GAT→AAT | Asp→Asn | |||||
| Tumor 5 | GAT→AAT | Asp→Asn | |||||
| Tumor 6 | nd∥ | ||||||
| Tumor 7 | GAT→CAT | Asp→His | |||||
| Tumor 8 | GAT→CAT | Asp→His | |||||
| 10 (F) | Control | wt | |||||
| Tumor 1 | GGA→GTA | Gly→Val | |||||
| Tumor 2 | GGA→GTA | Gly→Val | |||||
| Tumor 3 | wt | ||||||
| 11 (F) | Control | wt | |||||
| Tumor 1 | GAT→CAT | Asp→His | GGA→GTA | Gly→Val | |||
| Tumor 2 | GGA→GTA | Gly→Val | |||||
M-male; F-female.
The mutated base is underlined.
All controls were the DNAs extracted from the normal adjacent colon epithelial tissues of the same mice.
These samples were also used for exome sequencing.
This mutation could generate a few possible amino acid substitution.
There was no enough DNA or bad quaility of DNA extracted for sequencing.
Fig. 1. Summary of the mutations on the codon 32, 33 and 34 of Ctnnb1 in the PhIP-induced colon tumors in hCYP1A mice.
A. The nucleotide substitution/mutation in codon 32-34. B. The nucleotide and amino acid sequences of Ctnnb1 from codon 30 to 35. The common nucleotide sequences context in codon 32 and 34 are underlined, and the mutated positions are in bold.
Interestingly, Sanger sequencing result revealed that all 4 tumor samples used for exome sequencing carry Ctnnb1 codon 32 and 34 mutations, although exome sequence analysis result indicated no mutations in tumor No. 3. We examined the original exome sequencing data in tumor No. 3 for Ctnnb1, and found that this tumor actually carried codon 34 mutation, but the frequency of the DNA fragment carrying this mutation was low and it was recognized as false signal by analysis software (Supplemental Fig. 2). Indeed, this mutation, a conversion of GG to TT in exome sequence, was also true in Sanger sequencing result (Table 5). The Sanger sequencing result, however, could not determine whether the substation was a GGA to TTA or a mixture of GGA to GTA and TGA (a stop codon). The re-examination of original exome sequencing data excludes the possibility that the mutation of codon 34 generated a Stop codon.
There were three tumors, tumors 2 and 4 in mouse No.7 and tumor 1 in mouse No. 11, carrying more than one mutations (Table 5). Possibly, they were tumors that appeared as single tumors but consisted of tumor cells from different progenies in nearby location. Tumor 4 in mouse 1 carried C to T mutation on codon 33. This mutation might not be induced by PhIP since PhIP-DNA adduct is only on G. Because the DSS-induced colitis is necessary for this model, it is possible that mutation on codon 33 was caused by oxidative stress associated with colitis.
In addition, we sequenced the regions where the mutations of Dusp14 and Zbtb2 locate in 20 tumors in 5 male mice plus the adjacent normal colonic epithelium. For Dusp14, the results of 20 tumors and 5 matching controls showed that there was no mutation (Supplemental Table 4), indicating that the Dusp14 mutation found by exome-sequencing is a sequencing error. For Zbtb2, the result showed that the A to C mutation found in exome-sequencing were in all 20 tumors and 5 normal controls, suggesting that the hCYP1A mice used in our experiment carry a single nucleotide polymorphism (Supplemental Table 5) and this “mutation” is not associated with the PhIP/DSS-induced carcinogenesis. Indeed, the original exome-sequencing data showed that the sequencing coverage in this region of Zbtb2 in two normal control samples was very low (by only one sequenced oligonucleotide) and the C in the control samples was recognized as the sequencing error because the reference genome (mm9) is A at this position; thus the C in 4 exome-sequenced tumors were picked as the mutation.
Discussion
Mutations in APC and CTNNB1, resulting active Wnt signaling, are the most common events in the initiation of human CRC [2,6]. In this study, we characterized PhIP/DSS induced colon tumors in hCYP1A mice using exon sequencing and revealed that the activation mutation in Ctnnb1 is the most prevalent event. Mutations in Ctnnb1 are also found in azoxymethane (AOM)/DSS induced colon tumors in rodents [36,37]. AOM also induces mutations in Kras in rats (~70%) and mice (0-10%) as well as mutations in Apc in rats (~8%) [36]. The progression of tumorigenesis in our model is compatible to that of DSS treatment-induced colon tumor development in Apcmin/+ mouse; in which adenocarcinoma is induced in colon in about 5 weeks [38]. In the PhIP/DSS treated hCYP1A mice, colon adenocarcinoma also develops in about 5 weeks after DSS treatment [26]. This similarity suggests that PhIP treatment initiates colon carcinogenesis by attenuating the “gate-keeper” role of a controlled Wnt signaling.
Previous studies using different models established several potential mechanism of PhIP-induced carcinogenesis. Using cultured cells and rats, it was found that PhIP metabolite attacks DNA, forms dG-C8-PhIP adduct, and subsequently induces G to C mutation or G deletion, which was suggested to result in the expression of truncated APC protein or dominant active mutant Ctnnb1 and lead to the activation of Wnt signaling [39-42]. Notably, a -1G deletion in a G stretch such as 5’-GGGA-3’ in Apc gene was found in 4 out of 8 PhIP-induced F344 rat colon tumors, causing reading frame shift and truncation [43]. Such a -1G deletion was also reported in PhIP induced rat mammary gland tumor [42]. Mutations in Ctnnb1 gene were also reported in PhIP-induced rat and mouse colon tumors. An earlier report found Ctnnb1 gene mutations in 4 out of 7 PhIP-induced F344 rat colon tumor on codons 32, 34, 35, and 37 [44]. A more detailed study showed PhIP treatment in F344 rats results in Ctnnb1 mutations in 30% of aberrant crypt foci (ACF), 100% of adenoma (7 out of 7) and 50% of adenocarcinoma (6 out of 12); the mutations included those on codons 32, 33, and 34 that are found in human CRC and on codons 45, 47, and 56 that are not known in human CRC [45]. However, induction of tumor in rats require repeat intake of higher doses of PhIP (0.04% in diet for 40 weeks) [46]. A single dose of PhIP (i.e. 200 mg/kg BW) in combined with DSS-induced colitis was reported to induce colon tumors in mouse [47,48]. By targeted sequencing the exon 3 of Ctnnb1, mutations on codon 32 and 34 were found in 7 out 7 PhIP/DSS-induced CD-1 mouse tumors [48]. In the present study using exome-sequencing, a genome-wide approach, we did not found any G deletion in the sequenced region of Apc which was found in the PhIP-induced rat colon tumors. The overall incident with base deletion was very low (Table 3). Thus, the G-deletion may not play a significant role in PhIP-induced colon carcinogenesis in hCYP1A mice. Regarding Ctnnb1 mutations, we found mutations in over 92% of hCYP1A mouse colon tumors, consistent with previous finding that the activation mutations in Ctnnb1 are high frequent events in the PhIP/DSS-induced CD-1 mouse tumors [48].
In our study, a single dose of PhIP (200 mg/kg BW) followed by 7 days of 1.5% DSS treatment in the hCYP1A mice resulted in a tumor incidence of 100% with an average of 6 tumors per mouse [26]. The same treatment in the wildtype C57BL/6J did not produce any tumor in 24 weeks [26]; this result is different from the induction of low frequent tumorigenesis by the same dose of PhIP in the wildtype CD-1, C57BL/6J, and MSM/Ms mice without the human CYP1A gene reported previously [47,48]. The reason for this discrepancy is not known; it may be related to the dose of DSS and the diet used. In combination, these results suggest that the human CYP1A transgene in hCYP1A mice enhanced the activation of PhIP to the ultimate carcinogen, but it does not alter the mutation spectrum (i.e. mutations in Ctnnb1).
The consumption of high temperature cooked meat and fish containing PhIP and other HCA compounds increases the CRC risk [10,49,50]. PhIP-DNA adducts are commonly found in human colon biopsy samples [51]. Our result provides a molecular mechanism supporting the importance of PhIP in initiating human CRC. However, to what degree that PhIP contribute to human CRC or other cancers still needs be evaluated. First, active mutations of β-catenin/CTNNB1 are found in ≤5% of human CRCs [2,34], and the mutations on codons G32 and G34 of Ctnnb1 found in the present study may be similar to those in this type of CRC. Second, colitis-associated CRCs which the DSS-induced colitis models [52,53] are mainly driven by p53 mutation and loss of heterozygosity (LOH), found in ~50-80% human cases; in contrast, APC associated events are much less frequent (<33%) and seems not to be the earlier driver events [54]. Data of CTNNB1 mutation in colitis-associated CRC is not available because the prevalence is very low. It suggests that only a small fraction of human CRCs is caused by PhIP. Thus, the etiological role of PhIP in human CRC should be carefully evaluated with specific context. PhIP-DNA adducts are also found in other organs in human such as prostate [20,55] and the level is associated with the consumption of grilled meat [20,55]. In a rat model, PhIP-DNA adducts are also found in several organs such as colon, liver, pancreas, and lung [12,13,17,21,22]. Therefore, it is reasonable to suggest that the intake of PhIP and other HCAs could induce the similar mutation in other organs. CTNNB1 mutations were reported in 5% prostate cancer [56], 16-54% endometrial ovarian cancer [57], 3-44% hepatocellular carcinoma [58], 16-38 % adrenal tumors [59], and 85% desmoid tumors [60]. CTNNB1 was also found as one of the 13 significant mutated genes in a recent genome wide sequencing study on 183 lung cancers and its mutation seems not to co-occur with KRAS mutation [61]. We recently reported that PhIP induces prostate lesions (i.e. high-grade prostatic intraepithelial neoplasia) in hCYP1A mice [27]. Thus, it is highly likely that PhIP also induces carcinogenesis in other organs.
In summary, our study revealed that over 92% of PhIP/DSS-induced colon tumors in hCYP1A mice carry the dominant active mutation in Ctnnb1 gene. This result demonstrated that PhIP/DSS-induced colon carcinogenesis in hCYP1A mice is caused by the aberration of the gate-keeper of Wnt signaling, a cancer driver event in majority of human CRCs. The results indicate that our model in hCYP1A mice mimics human colorectal carcinogenesis is not only in the dietary etiology involving PhIP, but also in the aberrant activation of the Wnt singaling pathway as the driver.
Supplementary Material
Acknowledgement
We thank Dr. Guangxun Li for technical support and discussion. This work was supported by the National Institutes of Health (RO1CA120915, RO1 CA122474 and RO1 CA133021) as well as the Shared facilities funded by CA72720 and ES05022.
Abbreviations used
- AOM
azoxymethane
- CRC
colorectal cancer
- CYP
cytochrome P450
- DSS
dextrin sulfate sodium
- MCR
mutation cluster region
- MNU
N-methy-N-nitrosourea
- PhIP
2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer Statistics, 2014. CA Cancer J Clin. 2014;64(1):00–000. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Fearon ER. Molecular genetics of colorectal cancer. Annu Rev Pathol. 2011;6:479–507. doi: 10.1146/annurev-pathol-011110-130235. [DOI] [PubMed] [Google Scholar]
- 3.Johnson V, Volikos E, Halford SE, et al. Exon 3 beta-catenin mutations are specifically associated with colorectal carcinomas in hereditary non-polyposis colorectal cancer syndrome. Gut. 2005;54(2):264–267. doi: 10.1136/gut.2004.048132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487(7407):330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khare S, Verma M. Epigenetics of colon cancer. Methods Mol Biol. 2012;863:177–185. doi: 10.1007/978-1-61779-612-8_10. [DOI] [PubMed] [Google Scholar]
- 6.Jones S, Chen WD, Parmigiani G, et al. Comparative lesion sequencing provides insights into tumor evolution. Proc Natl Acad Sci U S A. 2008;105(11):4283–4288. doi: 10.1073/pnas.0712345105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Jr., Kinzler KW. Cancer genome landscapes. Science. 2013;339(6127):1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sutandyo N. Nutritional carcinogenesis. Acta Med Indones. 2012;42(1):36–42. [PubMed] [Google Scholar]
- 9.Anand P, Kunnumakkara AB, Sundaram C, et al. Cancer is a preventable disease that requires major lifestyle changes. Pharm Res. 2008;25(9):2097–2116. doi: 10.1007/s11095-008-9661-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vargas AJ, Thompson PA. Diet and nutrient factors in colorectal cancer risk. Nutr Clin Pract. 2012;27(5):613–623. doi: 10.1177/0884533612454885. [DOI] [PubMed] [Google Scholar]
- 11.Puangsombat K, Gadgil P, Houser TA, Hunt MC, Smith JS. Occurrence of heterocyclic amines in cooked meat products. Meat Sci. 2012;90(3):739–746. doi: 10.1016/j.meatsci.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Sugimura T, Wakabayashi K, Nakagama H, Nagao M. Heterocyclic amines: Mutagens/carcinogens produced during cooking of meat and fish. Cancer Sci. 2004;95(4):290–299. doi: 10.1111/j.1349-7006.2004.tb03205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagao M. A new approach to risk estimation of food-borne carcinogens--heterocyclic amines--based on molecular information. Mutat Res. 1999;431(1):3–12. doi: 10.1016/s0027-5107(99)00154-2. [DOI] [PubMed] [Google Scholar]
- 14.Felton JS, Knize MG, Shen NH, et al. The isolation and identification of a new mutagen from fried ground beef: 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP). Carcinogenesis. 1986;7(7):1081–1086. doi: 10.1093/carcin/7.7.1081. [DOI] [PubMed] [Google Scholar]
- 15.Lauber SN, Gooderham NJ. The cooked meat derived genotoxic carcinogen 2-amino-3-methylimidazo[4,5-b]pyridine has potent hormone-like activity: mechanistic support for a role in breast cancer. Cancer Res. 2007;67(19):9597–9602. doi: 10.1158/0008-5472.CAN-07-1661. [DOI] [PubMed] [Google Scholar]
- 16.Patterson AD, Gonzalez FJ, Idle JR. Xenobiotic metabolism: a view through the metabolometer. Chem Res Toxicol. 2010;23(5):851–860. doi: 10.1021/tx100020p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakagama H, Nakanishi M, Ochiai M. Modeling human colon cancer in rodents using a food-borne carcinogen, PhIP. Cancer Sci. 2005;96(10):627–636. doi: 10.1111/j.1349-7006.2005.00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friesen MD, Kaderlik K, Lin D, et al. Analysis of DNA adducts of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine in rat and human tissues by alkaline hydrolysis and gas chromatography/electron capture mass spectrometry: validation by comparison with 32P-postlabeling. Chem Res Toxicol. 1994;7(6):733–739. doi: 10.1021/tx00042a004. [DOI] [PubMed] [Google Scholar]
- 19.Ishibe N, Sinha R, Hein DW, et al. Genetic polymorphisms in heterocyclic amine metabolism and risk of colorectal adenomas. Pharmacogenetics. 2002;12(2):145–150. doi: 10.1097/00008571-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Tang D, Liu JJ, Rundle A, et al. Grilled meat consumption and PhIP-DNA adducts in prostate carcinogenesis. Cancer Epidemiol Biomarkers Prev. 2007;16(4):803–808. doi: 10.1158/1055-9965.EPI-06-0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dingley KH, Ubick EA, Chiarappa-Zucca ML, et al. Effect of dietary constituents with chemopreventive potential on adduct formation of a low dose of the heterocyclic amines PhIP and IQ and phase II hepatic enzymes. Nutr Cancer. 2003;46(2):212–221. doi: 10.1207/S15327914NC4602_15. [DOI] [PubMed] [Google Scholar]
- 22.Schut HA, Cummings DA, Smale MH, Josyula S, Friesen MD. DNA adducts of heterocyclic amines: formation, removal and inhibition by dietary components. Mutat Res. 1997;376(1-2):185–194. doi: 10.1016/s0027-5107(97)00042-0. [DOI] [PubMed] [Google Scholar]
- 23.Kimura S, Kawabe M, Yu A, et al. Carcinogenesis of the food mutagen PhIP in mice is independent of CYP1A2. Carcinogenesis. 2003;24(3):583–587. doi: 10.1093/carcin/24.3.583. [DOI] [PubMed] [Google Scholar]
- 24.Dragin N, Uno S, Wang B, Dalton TP, Nebert DW. Generation of ‘humanized’ hCYP1A1_1A2_Cyp1a1/1a2(−/−) mouse line. Biochem Biophys Res Commun. 2007;359(3):635–642. doi: 10.1016/j.bbrc.2007.05.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheung C, Ma X, Krausz KW, et al. Differential metabolism of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in mice humanized for CYP1A1 and CYP1A2. Chem Res Toxicol. 2005;18(9):1471–1478. doi: 10.1021/tx050136g. [DOI] [PubMed] [Google Scholar]
- 26.Cheung C, Loy S, Li GX, Liu AB, Yang CS. Rapid induction of colon carcinogenesis in CYP1A-humanized mice by 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine and dextran sodium sulfate. Carcinogenesis. 2011;32(2):233–239. doi: 10.1093/carcin/bgq235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li G, Wang H, Liu AB, et al. Dietary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine-induced prostate carcinogenesis in CYP1A-humanized mice. Cancer Prev Res (Phila) 2012;5(7):963–972. doi: 10.1158/1940-6207.CAPR-12-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hodis E, Watson IR, Kryukov GV, et al. A landscape of driver mutations in melanoma. Cell. 2012;150(2):251–263. doi: 10.1016/j.cell.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sentani K, Oue N, Naito Y, et al. Upregulation of HOXA10 in gastric cancer with the intestinal mucin phenotype: reduction during tumor progression and favorable prognosis. Carcinogenesis. 2012;33(5):1081–1088. doi: 10.1093/carcin/bgs121. [DOI] [PubMed] [Google Scholar]
- 30.Oyama T, Harigaya K, Sasaki N, et al. Mastermind-like 1 (MamL1) and mastermind-like 3 (MamL3) are essential for Notch signaling in vivo. Development. 2011;138(23):5235–5246. doi: 10.1242/dev.062802. [DOI] [PubMed] [Google Scholar]
- 31.Wu G, Xu G, Schulman BA, Jeffrey PD, Harper JW, Pavletich NP. Structure of a beta-TrCP1-Skp1-beta-catenin complex: destruction motif binding and lysine specificity of the SCF(beta-TrCP1) ubiquitin ligase. Mol Cell. 2003;11(6):1445–1456. doi: 10.1016/s1097-2765(03)00234-x. [DOI] [PubMed] [Google Scholar]
- 32.Provost E, McCabe A, Stern J, Lizardi I, D'Aquila TG, Rimm DL. Functional correlates of mutation of the Asp32 and Gly34 residues of beta-catenin. Oncogene. 2005;24(16):2667–2676. doi: 10.1038/sj.onc.1208346. [DOI] [PubMed] [Google Scholar]
- 33.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14(15):1837–1851. [PubMed] [Google Scholar]
- 34.Polakis P. The many ways of Wnt in cancer. Curr Opin Genet Dev. 2007;17(1):45–51. doi: 10.1016/j.gde.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 35.Minde DP, Anvarian Z, Rudiger SG, Maurice MM. Messing up disorder: how do missense mutations in the tumor suppressor protein APC lead to cancer? Mol Cancer. 2011;10(101):101. doi: 10.1186/1476-4598-10-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takahashi M, Wakabayashi K. Gene mutations and altered gene expression in azoxymethane-induced colon carcinogenesis in rodents. Cancer Sci. 2004;95(6):475–480. doi: 10.1111/j.1349-7006.2004.tb03235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takahashi M, Nakatsugi S, Sugimura T, Wakabayashi K. Frequent mutations of the beta-catenin gene in mouse colon tumors induced by azoxymethane. Carcinogenesis. 2000;21(6):1117–1120. [PubMed] [Google Scholar]
- 38.Tanaka T, Kohno H, Suzuki R, et al. Dextran sodium sulfate strongly promotes colorectal carcinogenesis in Apc(Min/+) mice: inflammatory stimuli by dextran sodium sulfate results in development of multiple colonic neoplasms. Int J Cancer. 2006;118(1):25–34. doi: 10.1002/ijc.21282. [DOI] [PubMed] [Google Scholar]
- 39.Andreassen A, Vikse R, Steffensen IL, Paulsen JE, Alexander J. Intestinal tumours induced by the food carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine in multiple intestinal neoplasia mice have truncation mutations as well as loss of the wild-type Apc(+) allele. Mutagenesis. 2001;16(4):309–315. doi: 10.1093/mutage/16.4.309. [DOI] [PubMed] [Google Scholar]
- 40.Burnouf D, Miturski R, Nagao M, et al. Early detection of 2-amino-1-methyl-6-phenylimidazo (4,5-b)pyridine(PhIP)-induced mutations within the Apc gene of rat colon. Carcinogenesis. 2001;22(2):329–335. doi: 10.1093/carcin/22.2.329. [DOI] [PubMed] [Google Scholar]
- 41.Andreassen A, Mollersen L, Vikse R, et al. One dose of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) or 2-amino-3-methylimidazo[4,5-f]quinoline (IQ) induces tumours in Min/+ mice by truncation mutations or LOH in the Apc gene. Mutat Res. 2002;517(1-2):157–166. doi: 10.1016/s1383-5718(02)00065-7. [DOI] [PubMed] [Google Scholar]
- 42.Okochi E, Watanabe N, Shimada Y, et al. Preferential induction of guanine deletion at 5′-GGGA-3′ in rat mammary glands by 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. Carcinogenesis. 1999;20(10):1933–1938. doi: 10.1093/carcin/20.10.1933. [DOI] [PubMed] [Google Scholar]
- 43.Kakiuchi H, Watanabe M, Ushijima T, et al. Specific 5′-GGGA-3′-->5′-GGA-3′ mutation of the Apc gene in rat colon tumors induced by 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. Proc Natl Acad Sci U S A. 1995;92(3):910–914. doi: 10.1073/pnas.92.3.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dashwood RH, Suzui M, Nakagama H, Sugimura T, Nagao M. High frequency of beta-catenin (ctnnb1) mutations in the colon tumors induced by two heterocyclic amines in the F344 rat. Cancer Res. 1998;58(6):1127–1129. [PubMed] [Google Scholar]
- 45.Tsukamoto T, Tanaka H, Fukami H, et al. More frequent beta-catenin gene mutations in adenomas than in aberrant crypt foci or adenocarcinomas in the large intestines of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP)-treated rats. Jpn J Cancer Res. 2000;91(8):792–796. doi: 10.1111/j.1349-7006.2000.tb01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakagama H, Ochiai M, Ubagai T, et al. A rat colon cancer model induced by 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine, PhIP. Mutat Res. 2002;506-507:137–144. doi: 10.1016/s0027-5107(02)00160-4. [DOI] [PubMed] [Google Scholar]
- 47.Nakanishi M, Tazawa H, Tsuchiya N, Sugimura T, Tanaka T, Nakagama H. Mouse strain differences in inflammatory responses of colonic mucosa induced by dextran sulfate sodium cause differential susceptibility to PhIP-induced large bowel carcinogenesis. Cancer Sci. 2007;98(8):1157–1163. doi: 10.1111/j.1349-7006.2007.00528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tanaka T, Suzuki R, Kohno H, Sugie S, Takahashi M, Wakabayashi K. Colonic adenocarcinomas rapidly induced by the combined treatment with 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine and dextran sodium sulfate in male ICR mice possess beta-catenin gene mutations and increases immunoreactivity for beta-catenin, cyclooxygenase-2 and inducible nitric oxide synthase. Carcinogenesis. 2005;26(1):229–238. doi: 10.1093/carcin/bgh292. [DOI] [PubMed] [Google Scholar]
- 49.Chan AT, Giovannucci EL. Primary prevention of colorectal cancer. Gastroenterology. 2010;138(6):2029–2043. e2010. doi: 10.1053/j.gastro.2010.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller PE, Lazarus P, Lesko SM, et al. Meat-related compounds and colorectal cancer risk by anatomical subsite. Nutr Cancer. 2013;65(2):202–226. doi: 10.1080/01635581.2013.756534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jonsson C, Stal P, Sjoqvist U, Akerlund JE, Lofberg R, Moller L. DNA adducts in normal colonic mucosa from healthy controls and patients with colon polyps and colorectal carcinomas. Mutagenesis. 2010;25(5):499–504. doi: 10.1093/mutage/geq033. [DOI] [PubMed] [Google Scholar]
- 52.Tanaka T. Colorectal carcinogenesis: Review of human and experimental animal studies. J Carcinog. 2009;8(5):5. doi: 10.4103/1477-3163.49014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clapper ML, Cooper HS, Chang WC. Dextran sulfate sodium-induced colitis-associated neoplasia: a promising model for the development of chemopreventive interventions. Acta Pharmacol Sin. 2007;28(9):1450–1459. doi: 10.1111/j.1745-7254.2007.00695.x. [DOI] [PubMed] [Google Scholar]
- 54.Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287(1):G7–17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 55.Tang D, Kryvenko ON, Wang Y, et al. 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP)-DNA adducts in benign prostate and subsequent risk for prostate cancer. Int J Cancer. 2013;133(4):961–971. doi: 10.1002/ijc.28092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chesire DR, Ewing CM, Sauvageot J, Bova GS, Isaacs WB. Detection and analysis of beta-catenin mutations in prostate cancer. Prostate. 2000;45(4):323–334. doi: 10.1002/1097-0045(20001201)45:4<323::aid-pros7>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 57.Bell DA. Origins and molecular pathology of ovarian cancer. Mod Pathol. 2005;18(Suppl 2)(2):S19–32. doi: 10.1038/modpathol.3800306. [DOI] [PubMed] [Google Scholar]
- 58.White BD, Chien AJ, Dawson DW. Dysregulation of Wnt/beta-catenin signaling in gastrointestinal cancers. Gastroenterology. 2012;142(2):219–232. doi: 10.1053/j.gastro.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Berthon A, Martinez A, Bertherat J, Val P. Wnt/beta-catenin signalling in adrenal physiology and tumour development. Mol Cell Endocrinol. 2012;351(1):87–95. doi: 10.1016/j.mce.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 60.Lazar AJ, Tuvin D, Hajibashi S, et al. Specific mutations in the beta-catenin gene (CTNNB1) correlate with local recurrence in sporadic desmoid tumors. Am J Pathol. 2008;173(5):1518–1527. doi: 10.2353/ajpath.2008.080475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Imielinski M, Berger AH, Hammerman PS, et al. Mapping the hallmarks of lung adenocarcinoma with massively parallel sequencing. Cell. 2012;150(6):1107–1120. doi: 10.1016/j.cell.2012.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.