Abstract
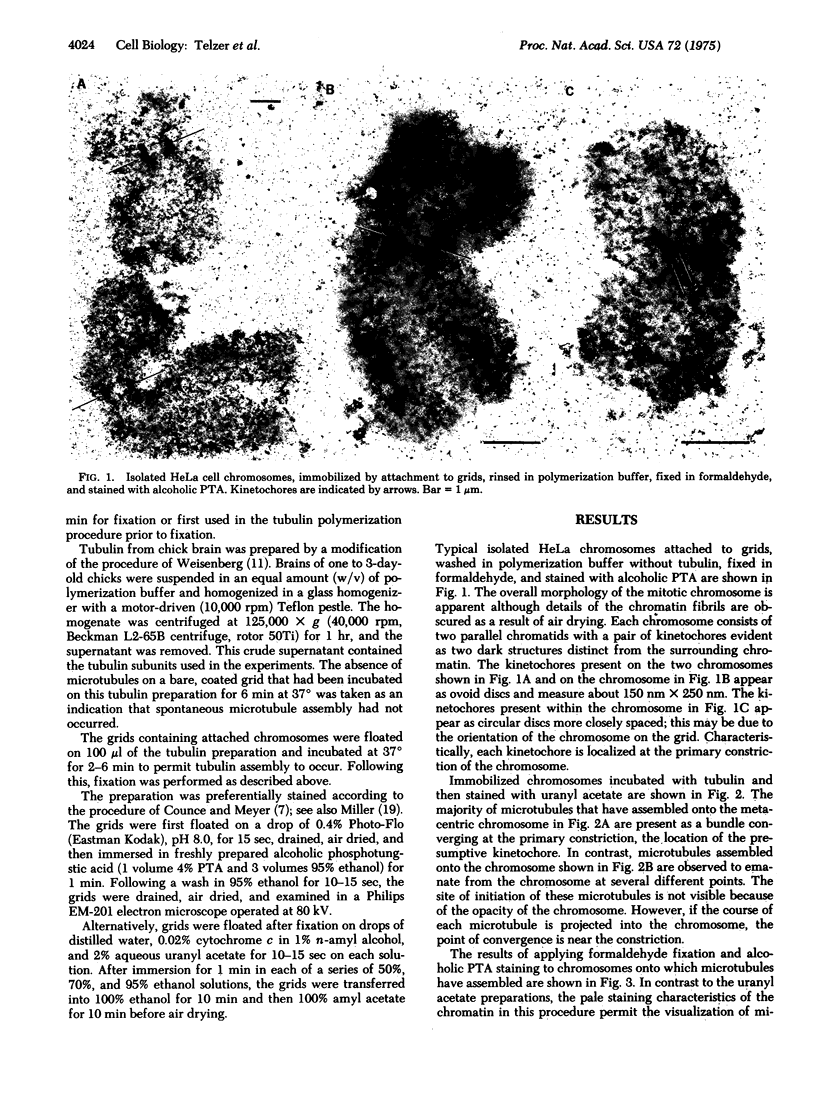
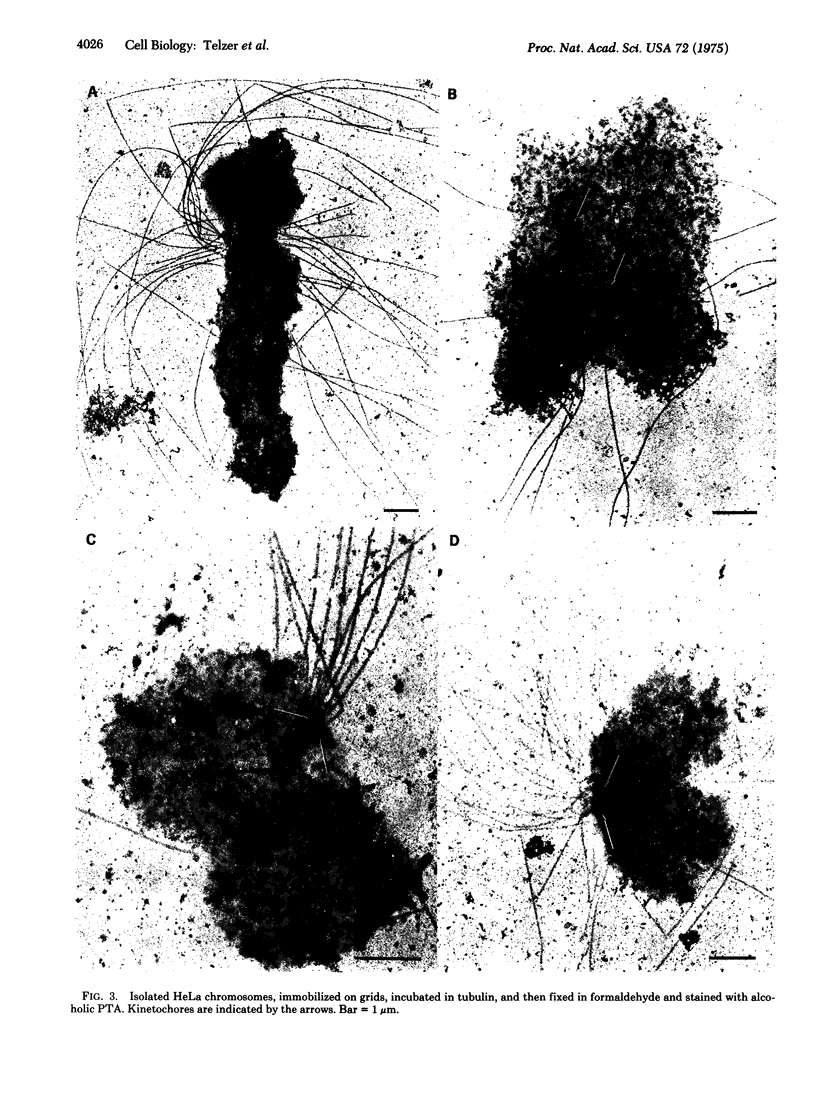
The kinetochores of isolated HeLa cell chromosomes attached to an electron microscope specimen grid, fixed in formaldehyde, and stained with alcoholic phosphotungstic acid are visible as dark, preferentially stained structures distinct from the chromatin with which they are associated. When unfixed chromosomes are immobilized by attachment to grids and incubated with chick brain tubulin, microtubules are observed to assemble onto the kinetochores. This demonstrates the competence of kinetochores in isolated chromosomes to act in vitro as microtubule assembly sites and suggests that they also possess this capacity in vivo. In addition, the results provide a possible means for isolating and characterizing kinetochores.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abuelo J. G., Moore D. E. The human chromosome. Electron microscopic observations on chromatin fiber organization. J Cell Biol. 1969 Apr;41(1):73–90. doi: 10.1083/jcb.41.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen C., Borisy G. G. Structural polarity and directional growth of microtubules of Chlamydomonas flagella. J Mol Biol. 1974 Dec 5;90(2):381–402. doi: 10.1016/0022-2836(74)90381-7. [DOI] [PubMed] [Google Scholar]
- Binder L. I., Dentler W. L., Rosenbaum J. L. Assembly of chick brain tubulin onto flagellar microtubules from Chlamydomonas and sea urchin sperm. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1122–1126. doi: 10.1073/pnas.72.3.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck R. C. Mitosis and meiosis in Rhodnius prolixus: the fine structure of the spindle and diffuse kinetochore. J Ultrastruct Res. 1967 Jun;18(5):489–501. doi: 10.1016/s0022-5320(67)80199-0. [DOI] [PubMed] [Google Scholar]
- Burkholder G. D., Okada T. A., Comings D. E. Whole mount electron microscopy of metaphase. I. Chromosomes and microtubules from mouse oocytes. Exp Cell Res. 1972 Dec;75(2):497–511. doi: 10.1016/0014-4827(72)90458-2. [DOI] [PubMed] [Google Scholar]
- Cande W. Z., Snyder J., Smith D., Summers K., McIntosh J. R. A functional mitotic spindle prepared from mammalian cells in culture. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1559–1563. doi: 10.1073/pnas.71.4.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comings D. E., Okada T. A. Holocentric chromosomes in Oncopeltus: kinetochore plates are present in mitosis but absent in meiosis. Chromosoma. 1972;37(2):177–192. doi: 10.1007/BF00284937. [DOI] [PubMed] [Google Scholar]
- Counce S. J., Meyer G. F. Differentiation of the synaptonemal complex and the kinetochore in Locusta spermatocytes studied by whole mount electron microscopy. Chromosoma. 1973 Nov 21;44(2):231–253. doi: 10.1007/BF00329119. [DOI] [PubMed] [Google Scholar]
- Dentler W. L., Granett S., Witman G. B., Rosenbaum J. L. Directionality of brain microtubule assembly in vitro. Proc Natl Acad Sci U S A. 1974 May;71(5):1710–1714. doi: 10.1073/pnas.71.5.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokelainen P. T. The ultrastructure and spatial organization of the metaphase kinetochore in mitotic rat cells. J Ultrastruct Res. 1967 Jul;19(1):19–44. doi: 10.1016/s0022-5320(67)80058-3. [DOI] [PubMed] [Google Scholar]
- Miller O. L., Jr, Beatty B. R. Visualization of nucleolar genes. Science. 1969 May 23;164(3882):955–957. doi: 10.1126/science.164.3882.955. [DOI] [PubMed] [Google Scholar]
- Moses M. J., Counce S. J. Electron microscopy of kinetochores in whole mount spreads of mitotic chromosomes from hela cells. J Exp Zool. 1974 Jul;189(1):115–120. doi: 10.1002/jez.1401890110. [DOI] [PubMed] [Google Scholar]
- Moses M. J., Counce S. J., Paulson D. F. Synaptonemal complex complement of man in spreads of spermatocytes, with details of the sex chromosome pair. Science. 1975 Jan 31;187(4174):363–365. [PubMed] [Google Scholar]
- ROTH L. E., DANIELS E. W. Electron microscopic studies of mitosis in amebae. II. The giant ameba Pelomyxa carolinensis. J Cell Biol. 1962 Jan;12:57–78. doi: 10.1083/jcb.12.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebhun L. I., Rosenbaum J., Lefebvre P., Smith G. Reversible restoration of the birefringence of cold-treated, isolated mitotic apparatus of surf clam eggs with chick brain tubulin. Nature. 1974 May 10;249(453):113–115. doi: 10.1038/249113a0. [DOI] [PubMed] [Google Scholar]
- Roos U. P. Light and electron microscopy of rat kangaroo cells in mitosis. II. Kinetochore structure and function. Chromosoma. 1973;41(2):195–220. doi: 10.1007/BF00319696. [DOI] [PubMed] [Google Scholar]
- Snell W. J., Dentler W. L., Haimo L. T., Binder L. I., Rosenbaum J. L. Assembly of chick brain tubulin onto isolated basal bodies of Chlamydomonas reinhardi. Science. 1974 Jul 26;185(4148):357–360. doi: 10.1126/science.185.4148.357. [DOI] [PubMed] [Google Scholar]
- Weisenberg R. C. Microtubule formation in vitro in solutions containing low calcium concentrations. Science. 1972 Sep 22;177(4054):1104–1105. doi: 10.1126/science.177.4054.1104. [DOI] [PubMed] [Google Scholar]
- Weisenberg R. C., Rosenfeld A. C. In vitro polymerization of microtubules into asters and spindles in homogenates of surf clam eggs. J Cell Biol. 1975 Jan;64(1):146–158. doi: 10.1083/jcb.64.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray W., Stubblefield E. A new method for the rapid isolation of chromosomes, mitotic apparatus, or nuclei from mammalian fibroblasts at near neutral pH. Exp Cell Res. 1970 Mar;59(3):469–478. doi: 10.1016/0014-4827(70)90656-7. [DOI] [PubMed] [Google Scholar]