Abstract
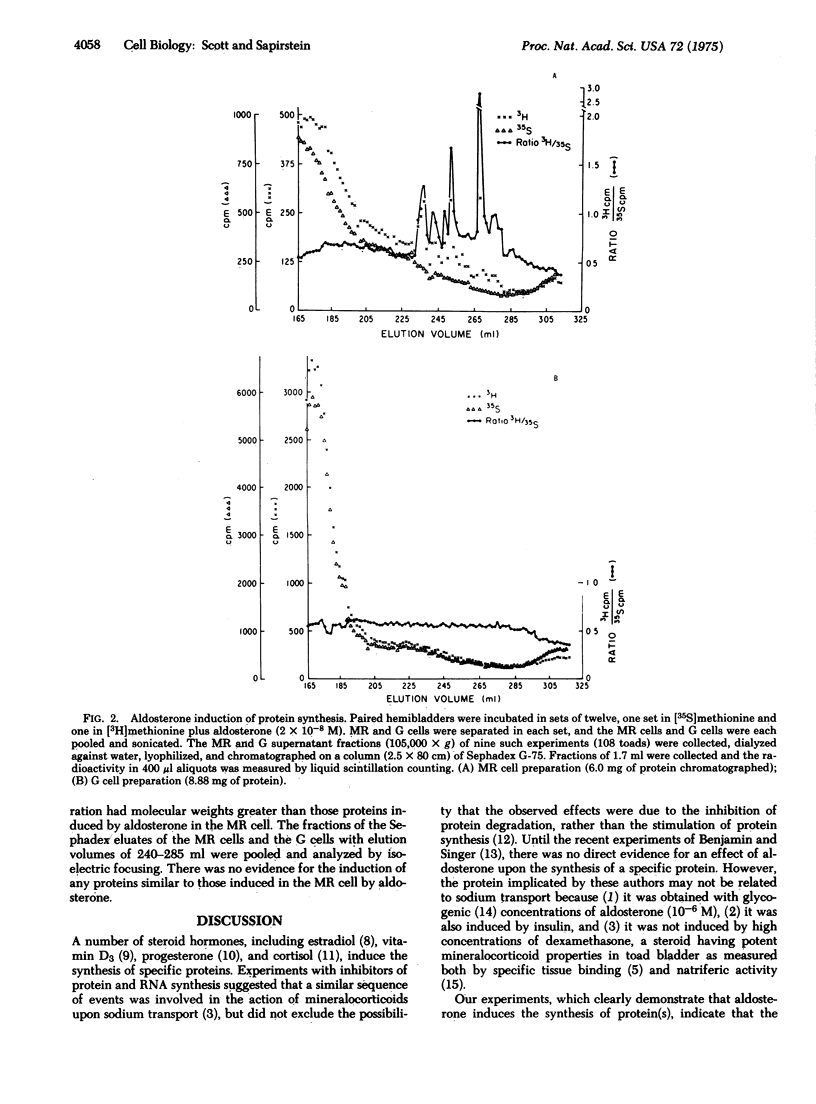
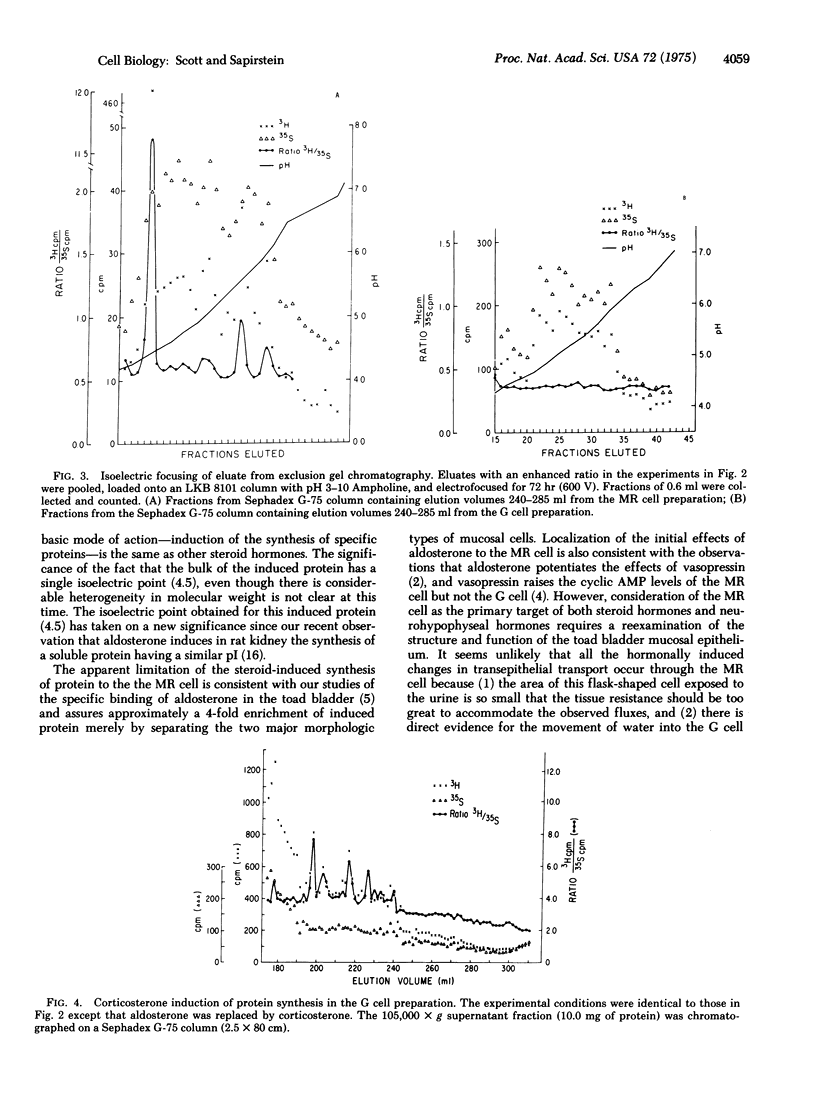
Aldosterone (the 8,11-hemiacetal 0f 11beta,21-dihydroxy-3,20-dioxo-4-pregnen-18-al) markedly stimulates sodium transport in a number of epithelial tissues. We have attempted to determine whether aldosterone induces the synthesis of specific protein(s) in the course of its action upon the toad urinary bladder. Paired hemibladders were incubated in media containing either [3H]methionine or [35S]methionine; aldosterone in physiologic concentrations was added to one bath and, after incubation, the intact "mitochondria-rich" (MR) and "granular" (G) mucosal cells were isolated. The ratio (3H/35S) was used to identify proteins whose synthesis was induced in the mucosal cells of the steroid-treated bladders. Using exclusion gel chromatography and isoelectric focusing, we identified several aldosterone-induced proteins in the supernatant (105,000 x g) fraction of the MR cell. None was evident in this fraction of the G cell. These proteins have apparent molecular weights ranging from 17,000 to 38,000 and the isoelectric point of the major component is 4.5. Corticosterone (11beta,21-dihydroxy-4-pregnene-3,20-dione) induced the synthesis of proteins in the G cells, but none of these proteins was similar in molecular weight to the aldosterone-induced proteins in the MR cell. Our findings support the hypothesis that aldosterone induces the synthesis of specific proteins and indicate that, in this tissue, these proteins are synthesized by the MR cell.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beato M., Kalimi M., Feigelson P. Correlation between glucocorticoid binding to specific liver cytosol receptors and enzyme induction in vivo. Biochem Biophys Res Commun. 1972 Jun 28;47(6):1464–1472. doi: 10.1016/0006-291x(72)90237-9. [DOI] [PubMed] [Google Scholar]
- Benjamin W. B., Singer I. Aldosterone-induced protein in toad urinary bladder. Science. 1974 Oct 18;186(4160):269–272. doi: 10.1126/science.186.4160.269. [DOI] [PubMed] [Google Scholar]
- CRABBE J. Stimulation of active sodium transport by the isolated toad bladder with aldosterone in vitro. J Clin Invest. 1961 Nov;40:2103–2110. doi: 10.1172/JCI104436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. P., Krauss M. R., Balis M. E., Dancis J. Metabolic cooperation in cell culture: studies of the mechanisms of cell interaction. J Cell Physiol. 1974 Oct;84(2):237–252. doi: 10.1002/jcp.1040840210. [DOI] [PubMed] [Google Scholar]
- Davis W. L., Goodman D. B., Martin J. H., Matthews J. L., Rasmussen H. Vasopressin-induced changes in the toad urinary bladder epithelial surface. J Cell Biol. 1974 May;61(2):544–547. doi: 10.1083/jcb.61.2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDELMAN I. S., BOGOROCH R., PORTER G. A. ON THE MECHANISM OF ACTION OF ALDOSTERONE ON SODIUM TRANSPORT: THE ROLE OF PROTEIN SYNTHESIS. Proc Natl Acad Sci U S A. 1963 Dec;50:1169–1177. doi: 10.1073/pnas.50.6.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handler J. S., Preston A. S., Orloff J. Effect of adrenal steroid hormones on the response of the toad's urinary bladder to vasopressin. J Clin Invest. 1969 May;48(5):823–833. doi: 10.1172/JCI106040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hax W. M., van Venrooij G. E., Vossenberg J. B. Cell communication: a cyclic AMP mediated phenomenon. J Membr Biol. 1974;19(3):253–266. doi: 10.1007/BF01869981. [DOI] [PubMed] [Google Scholar]
- Notides A., Gorski J. Estrogen-induced synthesis of a specific uterine protein. Proc Natl Acad Sci U S A. 1966 Jul;56(1):230–235. doi: 10.1073/pnas.56.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley B. W., McGuire W. L., Kohler P. O., Korenman S. G. Studies on the mechanism of steroid hormone regulation of synthesis of specific proteins. Recent Prog Horm Res. 1969;25:105–160. doi: 10.1016/b978-0-12-571125-8.50006-5. [DOI] [PubMed] [Google Scholar]
- Reel J. R., Kenney F. T. "Superinduction" of tyrosine transaminase in hepatoma cell cultures: differential inhibition of synthesis and turnover by actionomycin D. Proc Natl Acad Sci U S A. 1968 Sep;61(1):200–206. doi: 10.1073/pnas.61.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHULER W., DESAULLES P., MEIER R. Elektrocortin-Wirkung im Glykogentest. Experientia. 1954 Mar 15;10(3):142–142. doi: 10.1007/BF02158522. [DOI] [PubMed] [Google Scholar]
- Sapirstein V. S., Scott W. N. Binding of aldosterone by mitochondria-rich cells of the toad urinary bladder. Nature. 1975 Sep 18;257(5523):241–243. doi: 10.1038/257241a0. [DOI] [PubMed] [Google Scholar]
- Scott W. N., Sapirstein V. S., Yoder M. J. Partition of tissue functions in epithelia: localization of enzymes in "mitochondria-rich" cells of toad urinary bladder. Science. 1974 May 17;184(4138):797–800. doi: 10.1126/science.184.4138.797. [DOI] [PubMed] [Google Scholar]
- Wasserman R. H., Taylor A. N. Vitamin d3-induced calcium-binding protein in chick intestinal mucosa. Science. 1966 May 6;152(3723):791–793. doi: 10.1126/science.152.3723.791. [DOI] [PubMed] [Google Scholar]