Abstract
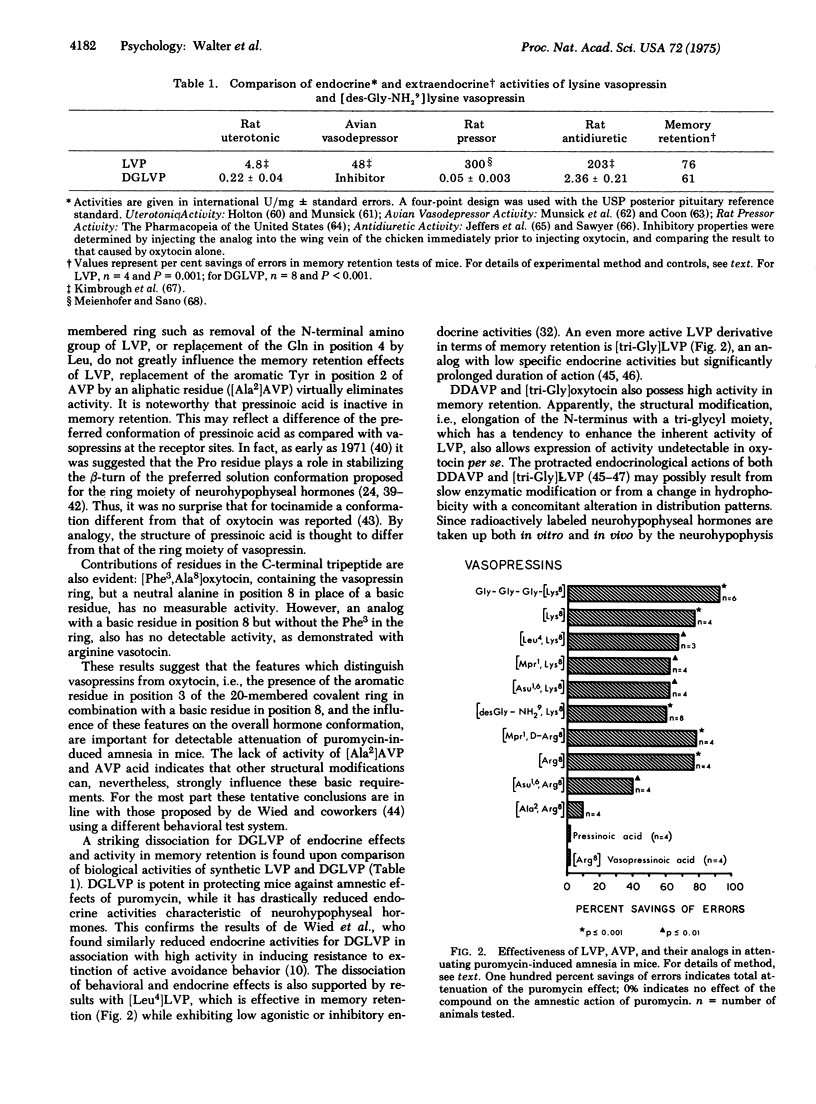
Neurohypophyseal hormones and several of their analogs, as well as N-terminal and C-terminal fragments, have been studied for their ability to attenuate puromycin-induced amnesia in mice. [8-Lysine]vasopressin, [8-arginine]vasopressin, and the analogs des-9-glycinamide-[8-lysine]vasopressin, [1-beta-mercaptopropionic acid, 8-lysine]vasopressin, [1,6-aminosuberic acid, 8-lysine]vasopressin, [4-leucine, 8-lysine]vasopressin, glycyl-glycyl-glycyl-[8-lysine]vasopressin, [1-beta-mercaptopropionic acid, 8-D-arginine]vasopressin, and [1,6-aminosuberic acid, 8-arginine]vasopressin are active. [8-Arginine]oxytocin as well as oxytocin and all of its other analogs tested are inactive with the striking exception of glycyl-glycyl-glycyl-oxytocin. The structural aspects of the neurohypophyseal hormones which appear to be important for significant activity in memory consolidation include the combination of a cyclic moiety containing the Tyr and Phe residues along with a basic residue in position 8. Another series of active compounds comprises C-terminal neurohypophyseal peptides and analogs thereof, including the naturally occurring Pro-Leu-Gly-NH2 and, most surprisingly, Leu-Gly-NH2, as well as its derivatives D-Leu-Gly-NH2 and the diketopiperazine, cyclo(-Leu-Gly-).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AROSKAR J. P., CHAN W. Y., STOUFFER J. E., SCHNEIDER C. H., MURTI V. V., DUVIGNEAUD V. RENAL EXCRETION AND TISSUE DISTRIBUTION OF RADIOACTIVITY AFTER ADMINISTRATION OF TRITIUM-LABELED OXYTOCIN TO RATS. Endocrinology. 1964 Feb;74:226–232. doi: 10.1210/endo-74-2-226. [DOI] [PubMed] [Google Scholar]
- Bohus B., Ader R., de Wied D. Effects of vasopressin on active and passive avoidance behavior. Horm Behav. 1972 Sep;3(3):191–197. doi: 10.1016/0018-506x(72)90031-1. [DOI] [PubMed] [Google Scholar]
- Bohus B., Gispen W. H., De Wied D. Effect of lysine vasopressin and ACTH 4-10 on conditioned avoidance behavior of hypophysectomized rats. Neuroendocrinology. 1973;11(3):137–143. doi: 10.1159/000122126. [DOI] [PubMed] [Google Scholar]
- Breslow E., Aanning H. L., Abrash L., Schmir M. Physical and chemical properties of the bovine neurophysins. J Biol Chem. 1971 Sep 10;246(17):5179–5188. [PubMed] [Google Scholar]
- Brewster A. I., Glasel J. A., Hruby V. J. Conformational studies on tocinamide and deaminotocinamide by 220 MHz nuclear magnetic resonance spectroscopy. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1470–1474. doi: 10.1073/pnas.69.6.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capra J. D., Cheng K. W., Friesen H. G., North W. G., Walter R. Evolution of neurophysin proteins: the partial sequence of human neurophysin-I. FEBS Lett. 1974 Sep 15;46(1):71–74. doi: 10.1016/0014-5793(74)80337-6. [DOI] [PubMed] [Google Scholar]
- Celis M. E., Hase S., Walter R. Structure-activity studies of MSH-release-inhibiting hormone. FEBS Lett. 1972 Nov 1;27(2):327–330. doi: 10.1016/0014-5793(72)80651-3. [DOI] [PubMed] [Google Scholar]
- Celis M. E., Taleisnik S., Walter R. Regulation of formation and proposed structure of the factor inhibiting the release of melanocyte-stimulating hormone. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1428–1433. doi: 10.1073/pnas.68.7.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wied D. Long term effect of vasopressin on the maintenance of a conditioned avoidance response in rats. Nature. 1971 Jul 2;232(5305):58–60. doi: 10.1038/232058a0. [DOI] [PubMed] [Google Scholar]
- Dyckes D. F., Ferger M. F., Du Vigneaud V., Chan W. Y. Synthesis and some of the pharmacological properties of (4-leucine)-8-lysine-vasopressin and (1-deamino,4-leucine)-8-lysine-vasopressin. J Med Chem. 1973 Jul;16(7):843–847. doi: 10.1021/jm00265a022. [DOI] [PubMed] [Google Scholar]
- Edwards B. A. Uptake of tritiated oxytocin into the neurohypophysis of the rat. J Endocrinol. 1971 Nov;51(3):607–608. doi: 10.1677/joe.0.0510607. [DOI] [PubMed] [Google Scholar]
- Ehrensing R. H., Kastin A. J. Melanocyte-stimulating hormone-release inhibiting hormone as a antidepressant. A pilot study. Arch Gen Psychiatry. 1974 Jan;30(1):63–65. doi: 10.1001/archpsyc.1974.01760070047007. [DOI] [PubMed] [Google Scholar]
- Flexner L. B., Flexner J. B., De La Haba G., Roberts R. B. Loss of memory as related to inhibition of cerebral protein synthesis. J Neurochem. 1965 Jul;12(7):535–541. doi: 10.1111/j.1471-4159.1965.tb04246.x. [DOI] [PubMed] [Google Scholar]
- Flexner L. B., Flexner J. B., Roberts R. B. Memory in mice analyzed with antibiotics. Antibiotics are useful to study stages of memory and to indicate molecular events which sustain memory. Science. 1967 Mar 17;155(3768):1377–1383. doi: 10.1126/science.155.3768.1377. [DOI] [PubMed] [Google Scholar]
- Hase S., Sakakibara S., Wahrenburg M., Kirchberger M., Schwartz I. L., Walter R. 1,6-Aminosuberic acid analogs of lysine- and arginine-vasopressin and -vasotocin. Synthesis and biological properties. J Am Chem Soc. 1972 May 17;94(10):3590–3600. doi: 10.1021/ja00765a056. [DOI] [PubMed] [Google Scholar]
- Havran R. T. Solid-phase synthesis of lysine vasopressin analogs: (1-beta-mercaptopropionic acid, 8-lysine)-vasopression and (1-beta-mercaptopropionic acid, 8-(epsilon-N-p-toluenesulfonyl)-lysine)-vasopressin. Experientia. 1973 May 15;29(5):520–521. doi: 10.1007/BF01926638. [DOI] [PubMed] [Google Scholar]
- KIMBROUGH R. D., Jr, CASH W. D., BRANDA L. A., CHAN W. Y., DU VIGNEAUD V. Synthesis and biological properties of 1-desamino-8-lysine-vasopressin. J Biol Chem. 1963 Apr;238:1411–1414. [PubMed] [Google Scholar]
- Kastin A. J., Barbeau A. Preliminary clinical studies with L-prolyl-L-leucyl-glycine amide in Parkinson's disease. Can Med Assoc J. 1972 Dec 9;107(11):1079–1081. [PMC free article] [PubMed] [Google Scholar]
- Kastin A. J., Dempsey G. L., LeBlanc B., Dyster-Aas K., Schally A. V. Extinction of an appetitive operant response after administration of MSH. Horm Behav. 1974 Jun;5(2):135–139. doi: 10.1016/0018-506x(74)90037-3. [DOI] [PubMed] [Google Scholar]
- King A. R., de Wied D. Localized behavioral effects of vasopressin on maintenance of an active avoidance response in rats. J Comp Physiol Psychol. 1974 Jun;86(6):1008–1018. doi: 10.1037/h0037636. [DOI] [PubMed] [Google Scholar]
- Kyncl J., Rezábek K., Kasafírek E., Pliska V., Rudinger J. 'Hormonogen' analogues of lysine vasopressin: rat pressor and antidiuretic activities. Eur J Pharmacol. 1974 Oct;28(2):294–301. doi: 10.1016/0014-2999(74)90282-9. [DOI] [PubMed] [Google Scholar]
- Lande S., Flexner J. B., Flexner L. B. Effect of corticotropin and desglycinamide 9 -lysine vasopressin on suppression of memory by puromycin. Proc Natl Acad Sci U S A. 1972 Mar;69(3):558–560. doi: 10.1073/pnas.69.3.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lande S., Witter A., de Wied D. Pituitary peptides. An octapeptide that stimulates conditioned avoidance acquisition in hypophysectomized rats. J Biol Chem. 1971 Apr 10;246(7):2058–2062. [PubMed] [Google Scholar]
- MUNSICK R. A. Effect of magnesium ion on the response of the rat uterus to neurohypophysial hormones and analogues. Endocrinology. 1960 Mar;6:451–457. doi: 10.1210/endo-66-3-451. [DOI] [PubMed] [Google Scholar]
- Meienhofer J., Trzeciak A., Havran R. T., Walter R. A solid-phase synthesis of (8-arginine)-vasopressin through a crystalline protected nonapeptide intermediate and biological properties of the hormone. J Am Chem Soc. 1970 Dec 2;92(24):7199–7202. doi: 10.1021/ja00727a031. [DOI] [PubMed] [Google Scholar]
- Merrifield R. B. Solid-phase peptide synthesis. Adv Enzymol Relat Areas Mol Biol. 1969;32:221–296. doi: 10.1002/9780470122778.ch6. [DOI] [PubMed] [Google Scholar]
- Miller L. H., Kastin A. J., Sandman C. A., Fink M., Van Veen W. J. Polypeptide influences on attention, memory and anxiety in man. Pharmacol Biochem Behav. 1974 Sep-Oct;2(5):663–668. doi: 10.1016/0091-3057(74)90035-5. [DOI] [PubMed] [Google Scholar]
- Nair R. M., Kastin A. J., Schally A. V. Isolation and structure of hypothalamic MSH release-inhibition hormone. Biochem Biophys Res Commun. 1971 Jun 18;43(6):1376–1381. doi: 10.1016/s0006-291x(71)80026-8. [DOI] [PubMed] [Google Scholar]
- Pliska V., Thorn N. A., Vilhardt H. In vitro uptake and breakdown of tritiated lysine-vasopressin by bovine neurohypophyseal and cortical tissue. Acta Endocrinol (Copenh) 1971 May;67(1):12–22. doi: 10.1530/acta.0.0670012. [DOI] [PubMed] [Google Scholar]
- Plotnikoff N. P., Kastin A. J., Anderson M. S., Schally A. V. Deserpidine antagonism by a tripeptide, L-prolyl-L-leucyglycinamide. Neuroendocrinology. 1973;11(1):67–71. doi: 10.1159/000122119. [DOI] [PubMed] [Google Scholar]
- Plotnikoff N. P., Kastin A. J., Anderson M. S., Schally A. V. Oxotremorine antagonism by a hypothalamic hormone, melanocyte-stimulating hormone release-inhibiting factor (MIF). Proc Soc Exp Biol Med. 1972 Jul;140(3):811–814. doi: 10.3181/00379727-140-36558. [DOI] [PubMed] [Google Scholar]
- Plotnikoff N. P., Kastin A. J. Oxotremorine antagonism by prolyl-leucyl-glycine-amide administered by different routes and with several anticholinergics. Pharmacol Biochem Behav. 1974 May;2(3):417–419. doi: 10.1016/0091-3057(74)90090-2. [DOI] [PubMed] [Google Scholar]
- Redding T. W., Kastin A. J., Nair R. M., Schally A. V. Distribution, half-life, and excretion of 14 C- and 3 H-labeled L-prolyl-L-leucyl-glycinamide in the rat. Neuroendocrinology. 1973;11(2):92–100. doi: 10.1159/000122121. [DOI] [PubMed] [Google Scholar]
- Redding T. W., Schally A. V. The distribution of radioactivity following the administration of labeled thyrotropin-releasing hormone (TRH) in rats and mice. Endocrinology. 1971 Oct;89(4):1075–1081. doi: 10.1210/endo-89-4-1075. [DOI] [PubMed] [Google Scholar]
- Rudinger J., Pliska V., Krejcí I. Oxytocin analogs in the analysis of some phases of hormone action. Recent Prog Horm Res. 1972;28:131–172. [PubMed] [Google Scholar]
- SAWYER W. H. Differences in the antidiuretic responses of rats to the intravenous administration of lysine and arginine vasopressins. Endocrinology. 1958 Nov;63(5):694–698. doi: 10.1210/endo-63-5-694. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Konishi S., Powell D., Leeman S. E., Otsuka M. Identification of the motoneuron-depolarizing peptide in bovine dorsal root as hypothalamic substance P. Brain Res. 1974 Jun 14;73(1):59–69. doi: 10.1016/0006-8993(74)91007-5. [DOI] [PubMed] [Google Scholar]
- Urry D. W., Walter R. Proposed conformation of oxytocin in solution. Proc Natl Acad Sci U S A. 1971 May;68(5):956–958. doi: 10.1073/pnas.68.5.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtin H., Sawyer W. H., Sokol H. W. Neurohypophysial principles in rats homozygous and heterozygous for hypothalamic diabetes insipidus (Brattleboro strain). Endocrinology. 1965 Oct;77(4):701–706. doi: 10.1210/endo-77-4-701. [DOI] [PubMed] [Google Scholar]
- Virkkunen P., Leppäluoto J., Lybeck H. The distribution and elimination of 125 I-labelled thyrotropin-releasing hormone in the mouse. Horm Metab Res. 1972 Nov;4(6):506–507. doi: 10.1055/s-0028-1097108. [DOI] [PubMed] [Google Scholar]
- Von Dreele P. H., Brewster A. I., Bovey F. A., Scheraga H. A., Ferger M. F., Du Vigneaud V. Nuclear magnetic resonance studies of lysine-vasopressin: structural constraints. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3088–3091. doi: 10.1073/pnas.68.12.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vávra I., Machová A., Holecek V., Cort J. H., Zaoral M., Sorm F. Effect of a synthetic analogue of vasopressin in animals and in patients with diabetes insipidus. Lancet. 1968 May 4;1(7549):948–952. doi: 10.1016/s0140-6736(68)90904-5. [DOI] [PubMed] [Google Scholar]
- Walter R., Glickson J. D., Schwartz I. L., Havran R. T., Meienhofer J., Urry D. W. Conformation of lysine vasopressin: a comparison with oxytocin. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1920–1924. doi: 10.1073/pnas.69.7.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter R., Griffiths E. C., Hooper K. C. Production of MSH-release-inhibiting hormone by a particulate preparation of hypothalami: mechanisms of oxytocin inactivation. Brain Res. 1973 Oct 12;60(2):449–457. doi: 10.1016/0006-8993(73)90802-0. [DOI] [PubMed] [Google Scholar]
- Wang S. S. Synthesis of desglycinamide lysine vasopressin and its behavioral activity in rats. Biochem Biophys Res Commun. 1972 Sep 26;48(6):1511–1515. doi: 10.1016/0006-291x(72)90885-6. [DOI] [PubMed] [Google Scholar]
- Willumsen N. B., Bie P. Tissue to plasma ratios of radioactivity in the rat hypothalamo-hypophyseal system after intraveous injection of 3H-lysine-vasopressin and 3H-mannitol. Acta Endocrinol (Copenh) 1969 Mar;60(3):389–400. doi: 10.1530/acta.0.0600389. [DOI] [PubMed] [Google Scholar]
- de Weid D., Bohus B., van Wimersma Greidanus T. B. Memory deficit in rats with hereditary diabetes insipidus. Brain Res. 1975 Feb 21;85(1):152–156. doi: 10.1016/0006-8993(75)91022-7. [DOI] [PubMed] [Google Scholar]
- de Wied D., Greven H. M., Lande S., Witter A. Dissociation of the behavioural and endocrine effects of lysine vasopressin by tryptic digestion. Br J Pharmacol. 1972 May;45(1):118–122. doi: 10.1111/j.1476-5381.1972.tb09582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wied D. Inhibitory effect of ACTH and related peptides on extinction of conditioned avoidance behavior in rats. Proc Soc Exp Biol Med. 1966 May;122(1):28–32. doi: 10.3181/00379727-122-31042. [DOI] [PubMed] [Google Scholar]
- van Wimersma T. B., Dogterom J., de Wied D. Intraventricular administration of anti-vasopressin serum inhibits. Life Sci. 1975 Feb 15;16(4):637–643. [PubMed] [Google Scholar]


