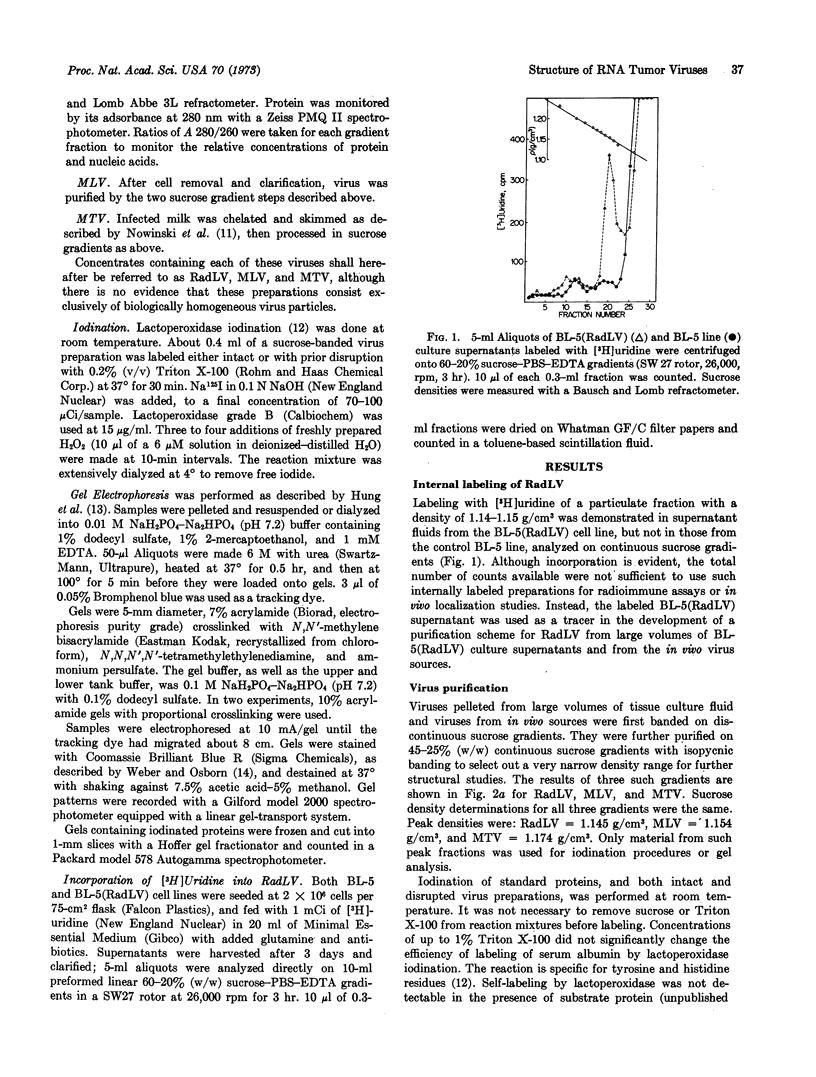
Abstract
Iodination by the noninvasive enzymatic lactoperoxidase technique has been used to study the enzyme-accessible and enzyme-inaccessible proteins of three oncorna viruses (radiation leukemia virus, Moloney leukemia virus, and mouse mammary tumor virus).
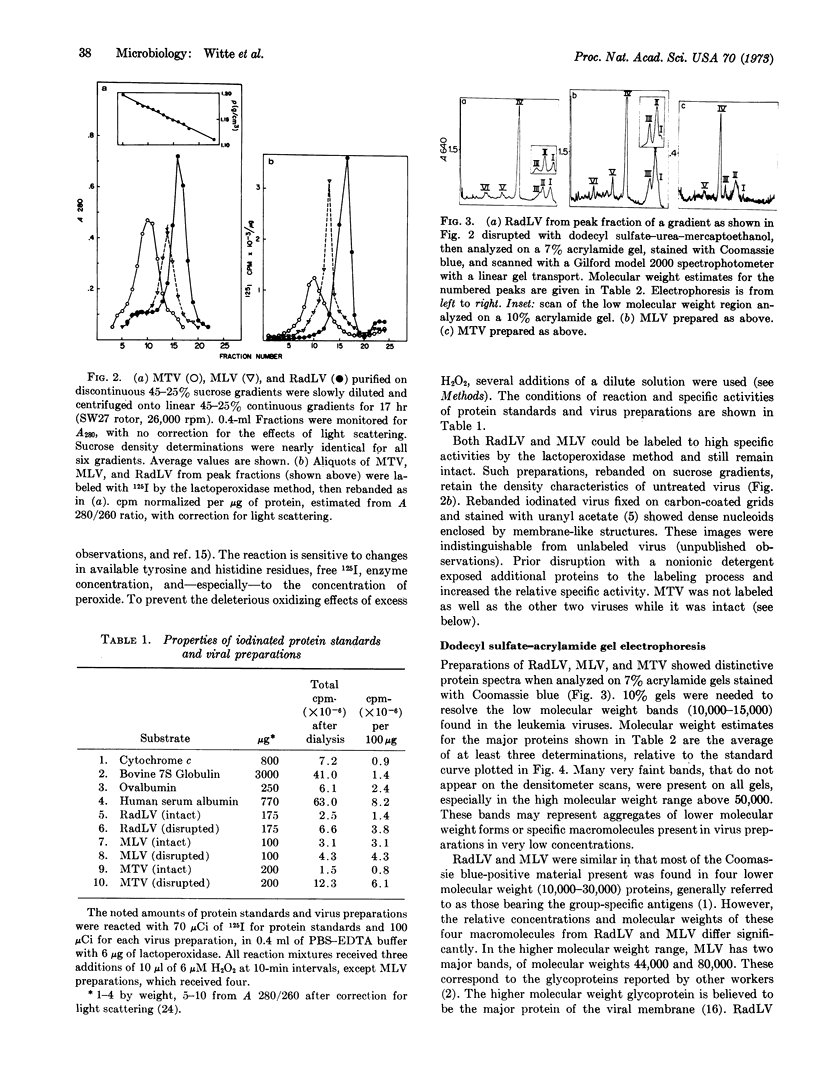
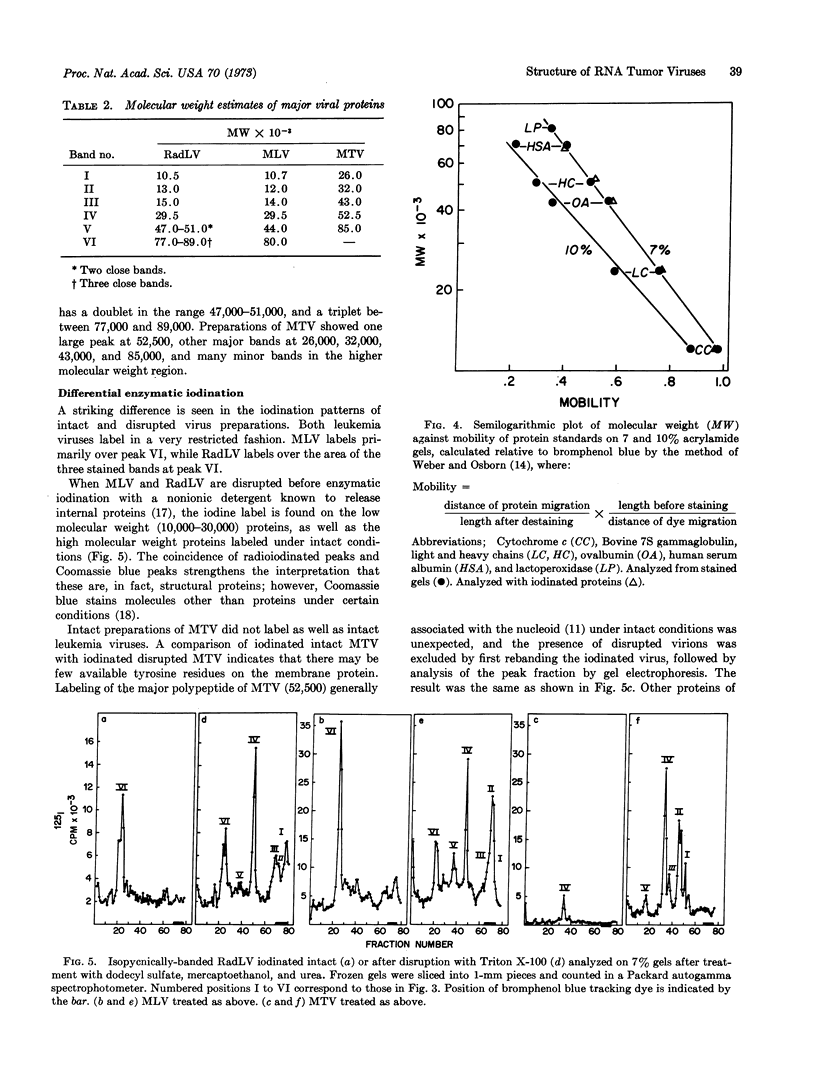
The number and relative molecular weight of proteins associated with virion preparations purified on sucrose gradients were characterized by scans of Coomassie blue-stained bands after dodecyl sulfate-polyacrylamide gel electrophoresis. Gel scans from the leukemia viruses are similar, each showing six distinct major protein bands on stained gels. The mammary tumor virus proteins by this analysis are not similar to those of the leukemia viruses.
Enzymatic iodination of intact virion preparations led to the solitary labeling of one of the major proteins of each virus—the 80,000-dalton protein of the leukemia viruses and the 52,500-dalton protein of the mammary tumor virus. These are tentatively positioned as surface (enzyme-accessible) moieties in the virions. Disruption of each virus with a nonionic nonionic detergent before enzymatic iodination led to the labeling of the remaining stainable bands. The four lower molecular weight bands of the leukemia viruses are tentatively positioned as internal (enzyme-inaccessible) components.
Keywords: radiation leukemia virus, Moloney leukemia virus, mouse mammary tumor virus, gel electrophoresis
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dreyer W. J., Papermaster D. S., Kühn H. On the absence of ubiquitous structural protein subunits in biological membranes. Ann N Y Acad Sci. 1972 Jun 20;195:61–74. [PubMed] [Google Scholar]
- Duesberg P. H., Martin G. S., Vogt P. K. Glycoprotein components of avian and murine RNA tumor viruses. Virology. 1970 Aug;41(4):631–646. doi: 10.1016/0042-6822(70)90428-9. [DOI] [PubMed] [Google Scholar]
- Fefer A., McCoy J. L., Glynn J. P. Antigenicity of a virus-induced murine sarcoma (Moloney). Cancer Res. 1967 May;27(5):962–967. [PubMed] [Google Scholar]
- Gerwin B. I., Todaro G. J., Zeve V., Scolnick E. M., Aaronson S. A. Separation of RNA-dependent DNA polymerase activity from the murine leukaemia virion. Nature. 1970 Oct 31;228(5270):435–438. doi: 10.1038/228435a0. [DOI] [PubMed] [Google Scholar]
- Hung P. P., Robinson H. L., Robinson W. S. Isolation and characterization of proteins from Rous sarcoma virus. Virology. 1971 Jan;43(1):251–266. doi: 10.1016/0042-6822(71)90243-1. [DOI] [PubMed] [Google Scholar]
- Kaplan H. S. On the natural history of the murine leukemias: presidential address. Cancer Res. 1967 Aug;27(8):1325–1340. [PubMed] [Google Scholar]
- LIEBERMAN M., KAPLAN H. S. Leukemogenic activity of filtrates from radiation-induced lymphoid tumors of mice. Science. 1959 Aug 14;130(3372):387–388. doi: 10.1126/science.130.3372.387. [DOI] [PubMed] [Google Scholar]
- Luftig R. B., Kilham S. S. An electron microscope study of Rauscher leukemia virus. Virology. 1971 Nov;46(2):277–297. doi: 10.1016/0042-6822(71)90030-4. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroni C. Structural proteins of Rauscher leukemia virus and Harvey sarcoma virus. Virology. 1972 Jan;47(1):1–7. doi: 10.1016/0042-6822(72)90232-2. [DOI] [PubMed] [Google Scholar]
- Nowinski R. C., Fleissner E., Sarkar N. H., Aoki T. Chromatographic separation and antigenic analysis of proteins of the oncornaviruses. II. Mammalian leukemia-sarcoma viruses. J Virol. 1972 Feb;9(2):359–366. doi: 10.1128/jvi.9.2.359-366.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oroszlan S., Foreman C., Kelloff G., Gilden R. V. The group-specific antigen and other structural proteins of hamster and mouse C-type viruses. Virology. 1971 Mar;43(3):665–674. doi: 10.1016/0042-6822(71)90290-x. [DOI] [PubMed] [Google Scholar]
- Parks W. P., Scolnick E. M. Radioimmunoassay of mammalian type-C viral proteins: interspecies antigenic reactivities of the major internal polypeptide. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1766–1770. doi: 10.1073/pnas.69.7.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins W. D., Karnovsky M. J., Unanue E. R. An ultrastructural study of lymphocytes with surface-bound immunoglobulin. J Exp Med. 1972 Feb 1;135(2):267–276. doi: 10.1084/jem.135.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poduslo J. F., Greenberg C. S., Glick M. C. Proteins exposed on the surface of mammalian membranes. Biochemistry. 1972 Jul 4;11(14):2616–2621. doi: 10.1021/bi00764a011. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Sarkar N. H., Nowinski R. C., Moore D. H. Helical nucleocapsid structure of the oncogenic ribonucleic acid viruses (oncornaviruses). J Virol. 1971 Oct;8(4):564–572. doi: 10.1128/jvi.8.4.564-572.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P., Haslam E. A. The polypeptides of influenza virus. V. Localization of polypeptides in the virion by iodination techniques. Virology. 1971 Dec;46(3):764–773. doi: 10.1016/0042-6822(71)90078-x. [DOI] [PubMed] [Google Scholar]
- Wallach D. F. The dispositions of proteins in the plasma membranes of animal cells: analytical approaches using controlled peptidolysis and protein labels. Biochim Biophys Acta. 1972 Feb 14;265(1):61–83. doi: 10.1016/0304-4157(72)90019-6. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]


