Abstract
The structure of a biologically active form of Renilla (sea pansy) luciferin has been elucidated; this structure, confirmed by total chemical synthesis, is 3,7-dihydro-2-methyl-6-(p-hydroxyphenyl)-8-benzylimidazo [1,2-a] pyrazin-3-one. In the natural compound the methyl group at the 2 position is replaced by an unknown, more complex group. For this reason the synthetic compound is 10% as active as the natural compound in producing light with Renilla luciferase. However, the spectral properties of the two compounds are identical. In addition the rates of the luminescent reaction with both compounds are similar, and the color of the light produced is identical in each case.
A compound isolated from the calcium-triggered photoprotein aequorin has been identified by Shimomura and Johnson [(1972) Biochemistry 11, 1602] to be 2-amino-3-benzyl-5-(p-hydroxyphenyl)pyrazine. This compound forms an integral part of the structure of Renilla luciferin. This, and other evidence, suggests that the structure elucidated for Renilla luciferin is a more general one associated with the luciferins of most, if not all, bioluminescent coelenterates.
Keywords: bioluminescence, pyrazine derivative, coelenterates
Full text
PDF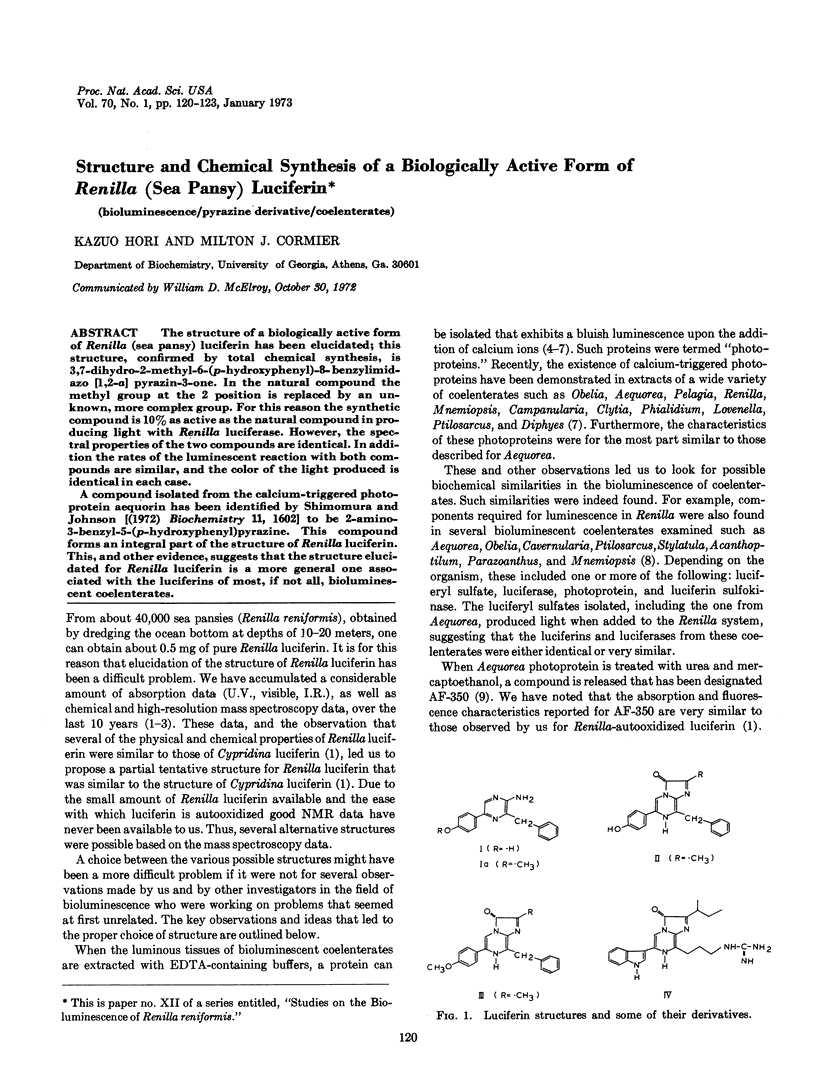

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cormier M. J., Hori K., Karkhanis Y. D. Studies on the bioluminescence of Renilla reniformis. VII. Conversion of luciferin into luciferyl sulfate by luciferin sulfokinase. Biochemistry. 1970 Mar 3;9(5):1184–1189. doi: 10.1021/bi00807a019. [DOI] [PubMed] [Google Scholar]
- Cormier M. J., Prichard P. M. An investigation of the mechanifm of the luminescent peroxidation of luminol by stopped flow techniques. J Biol Chem. 1968 Sep 25;243(18):4706–4714. [PubMed] [Google Scholar]
- DeLuca M., Dempsey M. E., Hori K., Wampler J. E., Cormier M. J. Mechanism of oxidative carbon dioxide production during Renilla reniformis bioluminescence. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1658–1660. doi: 10.1073/pnas.68.7.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori K., Cormier M. J. Studies on the bioluminescence of Renilla reniformis. VI. Some chemical properties and the tentative partial structure of luciferin. Biochim Biophys Acta. 1966 Dec 28;130(2):420–425. doi: 10.1016/0304-4165(66)90238-8. [DOI] [PubMed] [Google Scholar]
- Hori K., Nakano Y., Cormier M. J. Studies on the bioluminescence of Renilla reniformis. XI. Location of the sulfate group in luciferyl sulfate. Biochim Biophys Acta. 1972 Mar 16;256(3):638–644. doi: 10.1016/0005-2728(72)90199-5. [DOI] [PubMed] [Google Scholar]
- Karkhanis Y. D., Cormier M. J. Isolation and properties of Renilla reniformis luciferase, a low molecular weight energy conversion enzyme. Biochemistry. 1971 Jan 19;10(2):317–326. doi: 10.1021/bi00778a019. [DOI] [PubMed] [Google Scholar]
- Morin J. G., Hastings J. W. Biochemistry of the bioluminescence of colonial hydroids and other coelenterates. J Cell Physiol. 1971 Jun;77(3):305–312. doi: 10.1002/jcp.1040770304. [DOI] [PubMed] [Google Scholar]
- SHIMOMURA O., JOHNSON F. H., SAIGA Y. Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea. J Cell Comp Physiol. 1962 Jun;59:223–239. doi: 10.1002/jcp.1030590302. [DOI] [PubMed] [Google Scholar]
- Shimomura O., Johnson F. H. Properties of the bioluminescent protein aequorin. Biochemistry. 1969 Oct;8(10):3991–3997. doi: 10.1021/bi00838a015. [DOI] [PubMed] [Google Scholar]
- Shimomura O., Johnson F. H. Structure of the light-emitting moiety of aequorin. Biochemistry. 1972 Apr 25;11(9):1602–1608. doi: 10.1021/bi00759a009. [DOI] [PubMed] [Google Scholar]


