Abstract
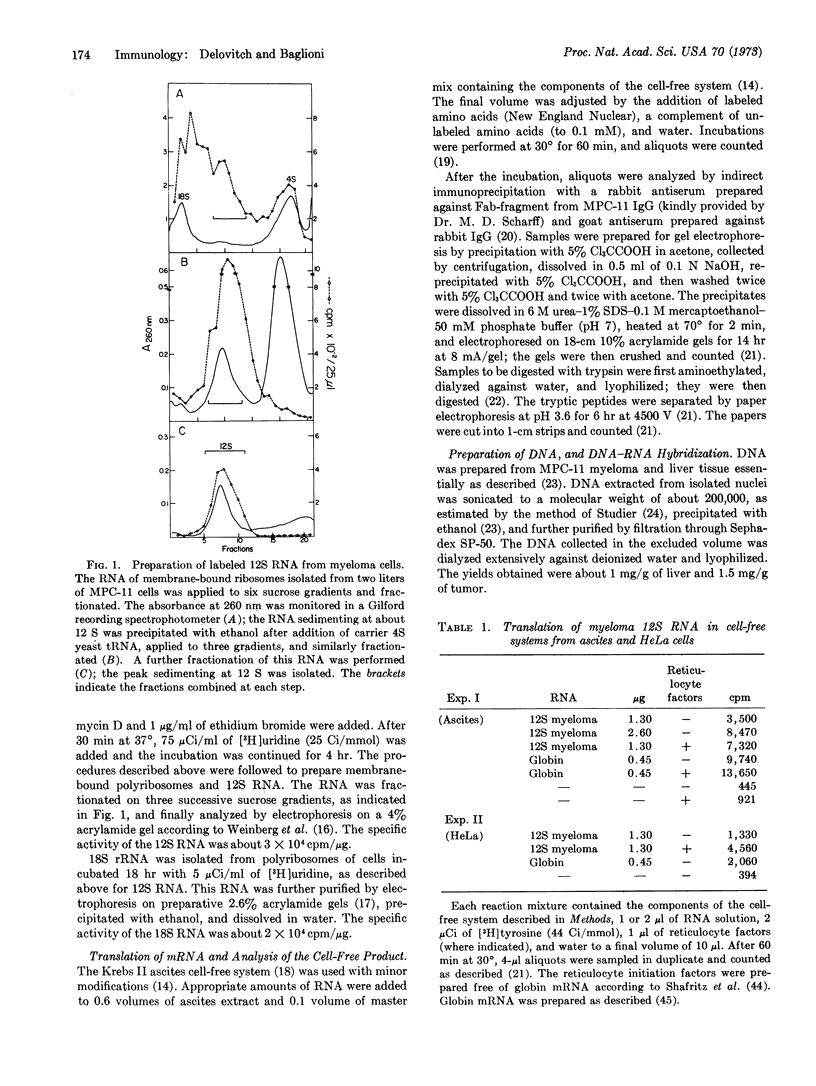
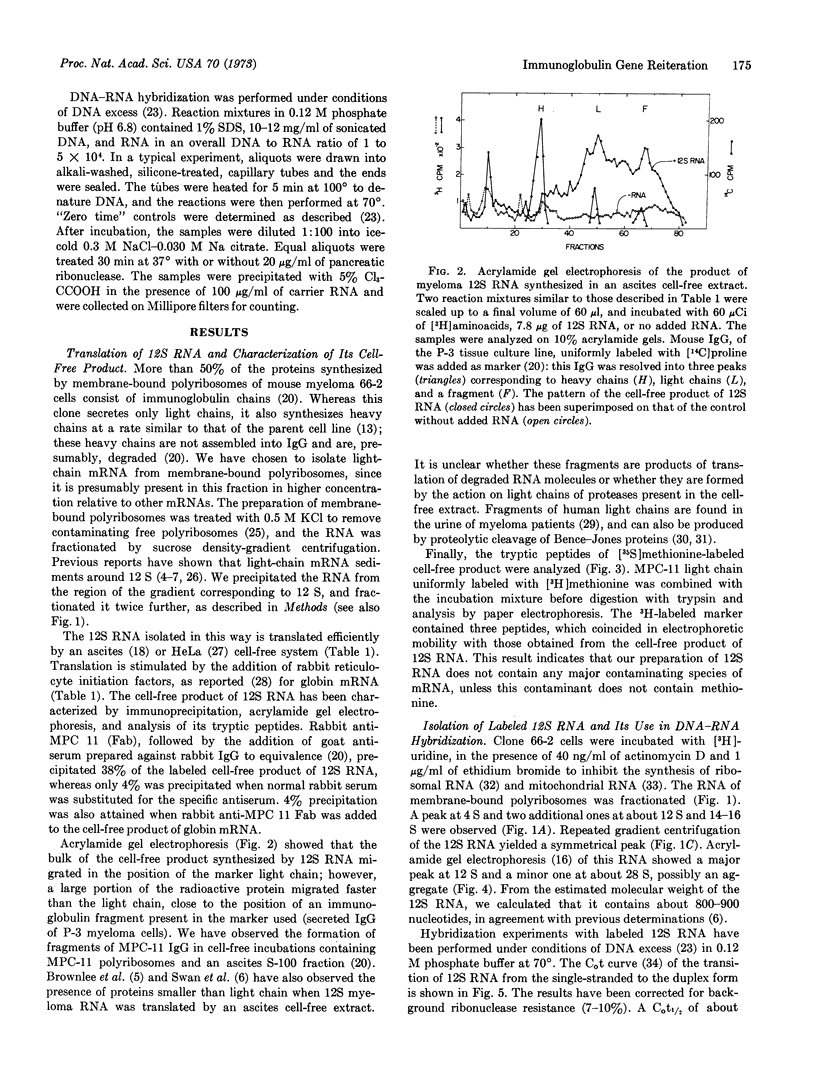
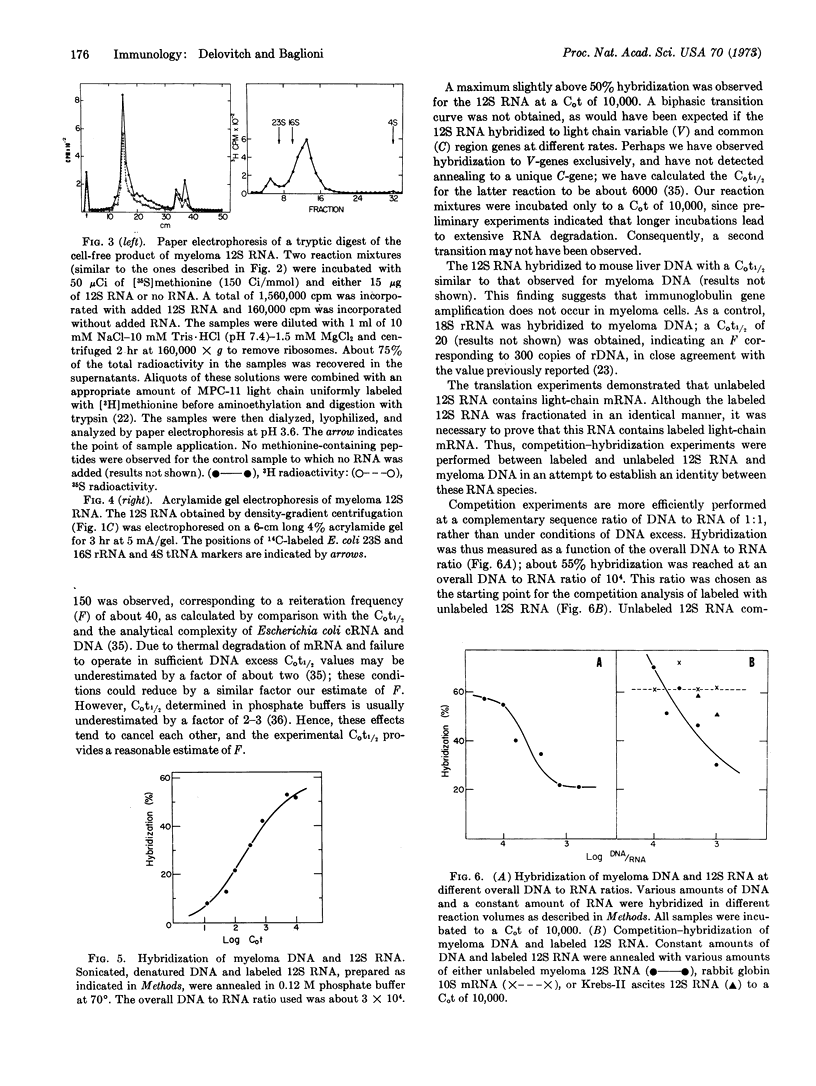
A 12S species of RNA has been isolated from membrane-bound polyribosomes of MPC-11 mouse myeloma cells. This 12S RNA is translated by an extract of Krebs-II mouse ascites cells into immunoglobulin light chain. Labeled 12S RNA has been prepared by incubation of myeloma cells with [3H]uridine in the presence of low concentrations of actinomycin D and ethidium bromide. This RNA has been hybridized under conditions of DNA excess to mouse myeloma DNA and to liver DNA. A C0t1/2 of about 150 has been obtained, corresponding to a reiteration frequency of about 40. Unlabeled 12S RNA competes with the labeled species in hybridization experiments, whereas globin mRNA or mouse ascites 12S RNA does not. It is suggested that 12S RNA hybridizes only to Vκ-genes of the same subclass, and that there may be several hundred genes coding for Vκ-regions of all subclasses in a mouse genome. Moreover, gene amplification in myeloma cells is not detected.
Keywords: 12S RNA, myeloma, protein synthesis, variable region
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baglioni C., Bleiberg I., Zauderer M. Assembly of membrane-bound polyribosomes. Nat New Biol. 1971 Jul 7;232(27):8–12. doi: 10.1038/newbio232008a0. [DOI] [PubMed] [Google Scholar]
- Baglioni C., La Via M., Ventruto V. A study of immunoglobulin structure. I. The fingerprinting of aminoethylated Bence-Jones proteins. Biochim Biophys Acta. 1965 Dec 16;111(2):479–484. doi: 10.1016/0304-4165(65)90057-7. [DOI] [PubMed] [Google Scholar]
- Bank A., Terada M., Metafora S., Dow L., Marks P. A. In vitro synthesis of DNA components of human genes for globins. Nat New Biol. 1972 Feb 9;235(58):167–169. doi: 10.1038/newbio235167a0. [DOI] [PubMed] [Google Scholar]
- Bishop J. O., Pemberton R., Baglioni C. Reiteration frequency of haemoglobin genes in the duck. Nat New Biol. 1972 Feb 23;235(60):231–234. doi: 10.1038/newbio235231a0. [DOI] [PubMed] [Google Scholar]
- Brega A., Vesco C. Ribonucleoprotein particles involved in HeLa mitochondrial protein synthesis. Nat New Biol. 1971 Feb 3;229(5):136–139. doi: 10.1038/newbio229136a0. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Brownlee G. G., Harrison T. M., Mathews M. B., Milstein C. Translation of messenger RNA for immunoglobulin light chains in a cell-free system from Krebs II ascites cells. FEBS Lett. 1972 Jun 15;23(2):244–248. doi: 10.1016/0014-5793(72)80352-1. [DOI] [PubMed] [Google Scholar]
- Coffino P., Scharff M. D. Rate of somatic mutation in immunoglobulin production by mouse myeloma cells. Proc Natl Acad Sci U S A. 1971 Jan;68(1):219–223. doi: 10.1073/pnas.68.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delovitch T. L., Davis B. K., Holme G., Sehon A. H. Isolation of messenger-like RNA from immunochemically separated polyribosomes. J Mol Biol. 1972 Aug 28;69(3):373–386. doi: 10.1016/0022-2836(72)90251-3. [DOI] [PubMed] [Google Scholar]
- Gally J. A., Edelman G. M. Somatic translocation of antibody genes. Nature. 1970 Jul 25;227(5256):341–348. doi: 10.1038/227341a0. [DOI] [PubMed] [Google Scholar]
- Harrison P. R., Hell A., Birnie G. D., Paul J. Evidence for single copies of globin genes in the mouse genome. Nature. 1972 Sep 22;239(5369):219–221. doi: 10.1038/239219a0. [DOI] [PubMed] [Google Scholar]
- Hood L. E., Potter M., McKean D. J. Immunoglobulin structure: amino terminal sequences of kappa chains from genetically similar mice (BALB/c). Science. 1970 Dec 11;170(3963):1207–1210. doi: 10.1126/science.170.3963.1207. [DOI] [PubMed] [Google Scholar]
- Hood L., Talmage D. W. Mechanism of antibody diversity: germ line basis for variability. Science. 1970 Apr 17;168(3929):325–334. doi: 10.1126/science.168.3929.325. [DOI] [PubMed] [Google Scholar]
- Jacobs-Lorena M., Baglioni C., Borun T. W. Translation of messenger RNA for histones from HeLa cells by a cell-free extract from mouse ascites tumor. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2095–2099. doi: 10.1073/pnas.69.8.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs-Lorena M., Baglioni C. Messenger RNA for globin in the postribosomal supernatant of rabbit reticulocytes. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1425–1428. doi: 10.1073/pnas.69.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson F. A., Peterson P. A., Berggard I. Properties of halves of immunoglobulin light chains. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1257–1263. doi: 10.1073/pnas.64.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedes L. H., Birnstiel M. L. Reiteration and clustering of DNA sequences complementary to histone messenger RNA. Nat New Biol. 1971 Apr 7;230(14):165–169. doi: 10.1038/newbio230165a0. [DOI] [PubMed] [Google Scholar]
- Laskov R., Scharff M. D. Synthesis, assembly, and secretion of gamma globulin by mouse myeloma cells. I. Adaptation of the Merwin plasma cell tumor-11 to culture, cloning, and characterization of gamma globulin subunits. J Exp Med. 1970 Mar 1;131(3):515–541. doi: 10.1084/jem.131.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews M., Korner A. Mammalian cell-free protein synthesis directed by viral ribonucleic acid. Eur J Biochem. 1970 Dec;17(2):328–338. doi: 10.1111/j.1432-1033.1970.tb01170.x. [DOI] [PubMed] [Google Scholar]
- McCarthy B. J., Church R. B. The specificity of molecular hybridization reactions. Annu Rev Biochem. 1970;39:131–150. doi: 10.1146/annurev.bi.39.070170.001023. [DOI] [PubMed] [Google Scholar]
- McCarthy D., Hawkes S. P., Lander D. E. Small scale preparative gel electrophoresis of ribonucleic acids. Biochim Biophys Acta. 1972 Aug 25;277(2):301–305. doi: 10.1016/0005-2787(72)90412-1. [DOI] [PubMed] [Google Scholar]
- McDowell M. J., Joklik W. K., Villa-Komaroff L., Lodish H. F. Translation of reovirus messenger RNAs synthetesized in vitro into reovirus polypeptides by several mammalian cell-free extracts. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2649–2653. doi: 10.1073/pnas.69.9.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melli M., Whitfield C., Rao K. V., Richardson M., Bishop J. O. DNA-RNA hybridization in vast DNA excess. Nat New Biol. 1971 May 5;231(18):8–12. [PubMed] [Google Scholar]
- Metafora S., Terada M., Dow L. W., Marks P. A., Bank A. Increased efficiency of exogenous messenger RNA translation in a Krebs ascites cell lysate. Proc Natl Acad Sci U S A. 1972 May;69(5):1299–1303. doi: 10.1073/pnas.69.5.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISHIMURA S., NOVELLI G. D. AMINO ACID ACCEPTOR ACTIVITY OF ENZYMICALLY ALTERED SOLUBLE RNA FROM ESCHERICHIA COLI. Biochim Biophys Acta. 1964 Apr 27;80:574–586. doi: 10.1016/0926-6550(64)90302-0. [DOI] [PubMed] [Google Scholar]
- Perry R. P. THE CELLULAR SITES OF SYNTHESIS OF RIBOSOMAL AND 4S RNA. Proc Natl Acad Sci U S A. 1962 Dec;48(12):2179–2186. doi: 10.1073/pnas.48.12.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W. K., Newman J. F. Use of low concentrations of actinomycin D in the study of RNA synthesis in Ehrlich ascites cells. J Mol Biol. 1966 Sep;20(1):63–73. doi: 10.1016/0022-2836(66)90117-3. [DOI] [PubMed] [Google Scholar]
- Ross J., Aviv H., Scolnick E., Leder P. In vitro synthesis of DNA complementary to purified rabbit globin mRNA (RNA-dependent DNA polymerase-reticulocyte-hemoglobin-density gradient centrifugation-oligo(dT) primer). Proc Natl Acad Sci U S A. 1972 Jan;69(1):264–268. doi: 10.1073/pnas.69.1.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Solomon A., McLaughlin C. L. Bence-Jones proteins and light chains of immunoglobulins. I. Formation and characterization of amino-terminal (variant) and carboxyl-terminal (constant) halves. J Biol Chem. 1969 Jun 25;244(12):3393–3404. [PubMed] [Google Scholar]
- Stavnezer J., Huang R. C. Synthesis of a mouse immunoglobulin light chain in a rabbit reticulocyte cell-free system. Nat New Biol. 1971 Apr 7;230(14):172–176. doi: 10.1038/newbio230172a0. [DOI] [PubMed] [Google Scholar]
- Stevens R. H., Williamson A. R. Specific IgG mRNA molecules from myeloma cells in heterogeneous nuclear and cytoplasmic RNA containing poly-A. Nature. 1972 Sep 15;239(5368):143–146. doi: 10.1038/239143a0. [DOI] [PubMed] [Google Scholar]
- Storb U. Quantitation of immunoglobulin genes by nucleic acid hybridization with RNA from myeloma and spleen microsomes. J Immunol. 1972 Mar;108(3):755–764. [PubMed] [Google Scholar]
- Swan D., Aviv H., Leder P. Purification and properties of biologically active messenger RNA for a myeloma light chain. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1967–1971. doi: 10.1073/pnas.69.7.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma I. M., Temple G. F., Fan H., Baltimore D. In vitro synthesis of DNA complementary to rabbit reticulocyte 10S RNA. Nat New Biol. 1972 Feb 9;235(58):163–167. doi: 10.1038/newbio235163a0. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A., Loening U., Willems M., Penman S. Acrylamide gel electrophoresis of HeLa cell nucleolar RNA. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1088–1095. doi: 10.1073/pnas.58.3.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T. T., Kabat E. A. An analysis of the sequences of the variable regions of Bence Jones proteins and myeloma light chains and their implications for antibody complementarity. J Exp Med. 1970 Aug 1;132(2):211–250. doi: 10.1084/jem.132.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylber E., Vesco C., Penman S. Selective inhibition of the synthesis of mitochondria-associated RNA by ethidium bromide. J Mol Biol. 1969 Aug 28;44(1):195–204. doi: 10.1016/0022-2836(69)90414-8. [DOI] [PubMed] [Google Scholar]


