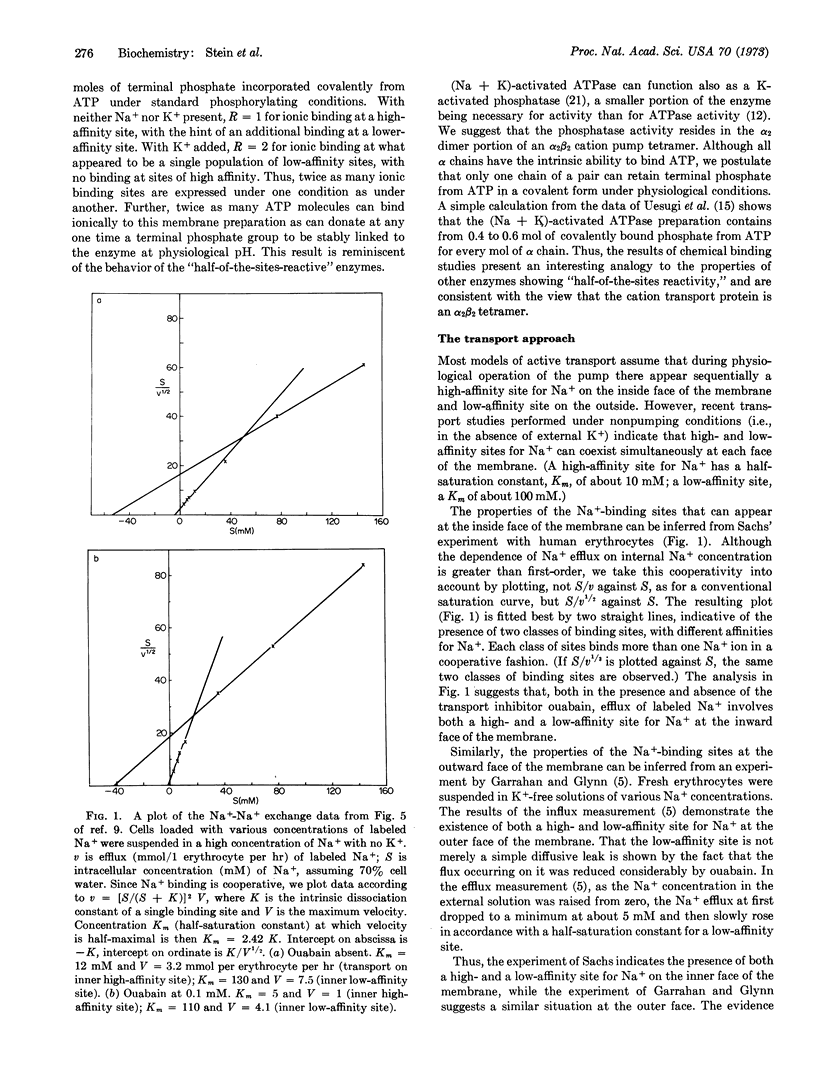
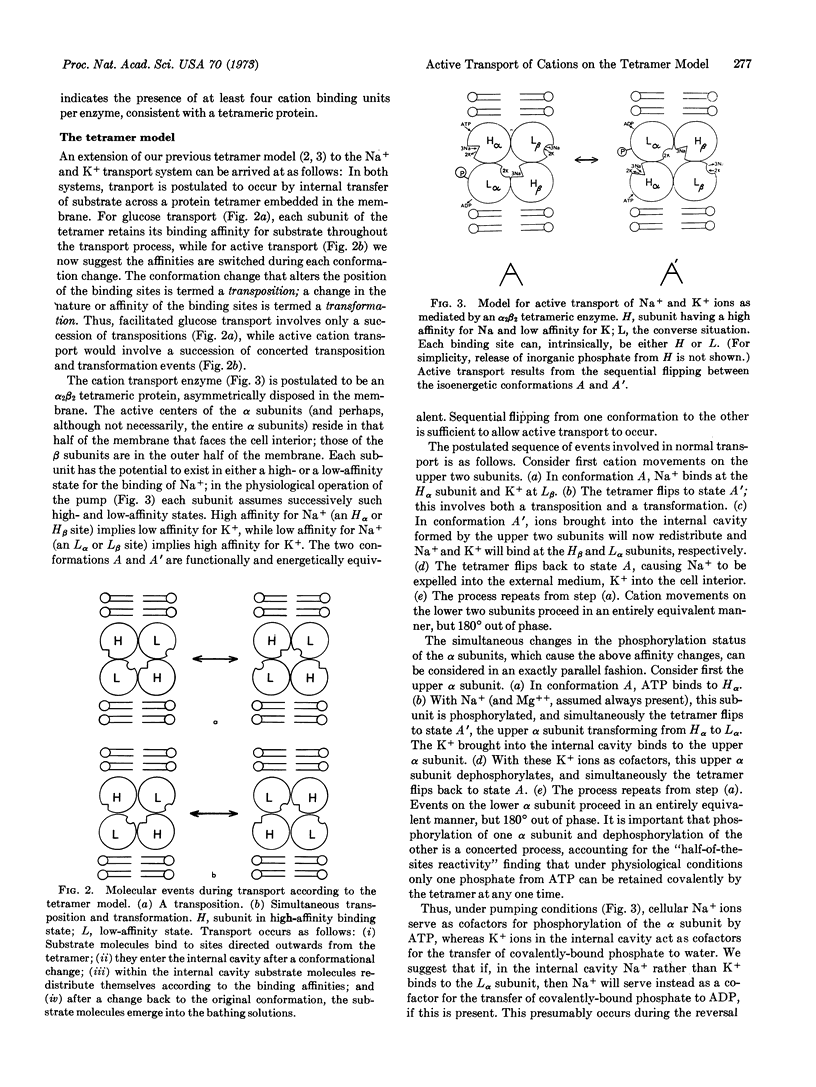
Abstract
A new model is proposed for the system that actively transports sodium and potassium ions across animal-cell membranes. The model is based on the physical and chemical properties of transport-associated adenosine triphosphatase (EC 3.6.1.3) and on the kinetics of ion movements mediated by the system. Transport is postulated to occur by internal transfer of cations across a protein tetramer embedded in the cell membrane. The protein tetramer can exist in either of two forms of identical energy; transport occurs as a result of the sequential “flipping” from one conformation to the other. The conformation change results in the interchanging of the affinities of cation-binding sites associated with different sub-units of the tetramer, with a concomitant splitting of adenosine triphosphate.
Keywords: cell membranes, adenosine triphosphatase, transport kinetics, cation pumping, protein conformation changes
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Applebury M. L., Johnson B. P., Coleman J. E. Phosphate binding to alkaline phosphatase. Metal ion dependence. J Biol Chem. 1970 Oct 10;245(19):4968–4976. [PubMed] [Google Scholar]
- Garrahan P. J., Glynn I. M. Facftors affecting the relative magnitudes of the sodium:potassium and sodium:sodium exchanges catalysed by the sodium pump. J Physiol. 1967 Sep;192(1):189–216. doi: 10.1113/jphysiol.1967.sp008296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrahan P. J., Glynn I. M. The behaviour of the sodium pump in red cells in the absence of external potassium. J Physiol. 1967 Sep;192(1):159–174. doi: 10.1113/jphysiol.1967.sp008294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrahan P. J., Glynn I. M. The incorporation of inorganic phosphate into adenosine triphosphate by reversal of the sodium pump. J Physiol. 1967 Sep;192(1):237–256. doi: 10.1113/jphysiol.1967.sp008298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Lew V. L., Lüthi U. Reversal of the potassium entry mechanism in red cells, with and without reversal of the entire pump cycle. J Physiol. 1970 Apr;207(2):371–391. doi: 10.1113/jphysiol.1970.sp009067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegyvary C., Post R. L. Binding of adenosine triphosphate to sodium and potassium ion-stimulated adenosine triphosphatase. J Biol Chem. 1971 Sep 10;246(17):5234–5240. [PubMed] [Google Scholar]
- Kepner G. R., Macey R. I. Membrane enzyme systems. Molecular size determinations by radiation inactivation. Biochim Biophys Acta. 1968 Sep 17;163(2):188–203. doi: 10.1016/0005-2736(68)90097-7. [DOI] [PubMed] [Google Scholar]
- Kyte J. Phosphorylation of a purified (Na + +K + ) adenosine triphosphatase. Biochem Biophys Res Commun. 1971 Jun 18;43(6):1259–1265. doi: 10.1016/s0006-291x(71)80008-6. [DOI] [PubMed] [Google Scholar]
- Kyte J. Purification of the sodium- and potassium-dependent adenosine triphosphatase from canine renal medulla. J Biol Chem. 1971 Jul 10;246(13):4157–4165. [PubMed] [Google Scholar]
- Lazdunski M., Petitclerc C., Chappelet D., Lazdunski C. Flip-flop mechanisms in enzymology. A model: the alkaline phosphatase of Escherichia coli. Eur J Biochem. 1971 May 11;20(1):124–139. doi: 10.1111/j.1432-1033.1971.tb01370.x. [DOI] [PubMed] [Google Scholar]
- Levitzki A., Stallcup W. B., Koshland D. E., Jr Half-of-the-sites reactivity and the conformational states of cytidine triphosphate synthetase. Biochemistry. 1971 Aug 31;10(18):3371–3378. doi: 10.1021/bi00794a009. [DOI] [PubMed] [Google Scholar]
- Lieb W. R., Stein W. D. Carrier and non-carrier models for sugar transport in the human red blood cell. Biochim Biophys Acta. 1972 Apr 18;265(2):187–207. doi: 10.1016/0304-4157(72)90002-0. [DOI] [PubMed] [Google Scholar]
- Lieb W. R., Stein W. D. New theory for glucose transport across membranes. Nat New Biol. 1971 Mar 24;230(12):108–109. doi: 10.1038/newbio230108a0. [DOI] [PubMed] [Google Scholar]
- Lieb W. R., Stein W. D. Quantitative predictions of a noncarrier model for glucose transport across the human red cell membrane. Biophys J. 1970 Jul;10(7):585–609. doi: 10.1016/s0006-3495(70)86322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao M., Nagano K., Nakao T., Mizuno N., Tashima Y., Fujita M., Maeda H., Matsudaira H. Molecular weight of Na, K-ATPase approximated by the radiation inactivation method. Biochem Biophys Res Commun. 1967 Nov 30;29(4):588–592. doi: 10.1016/0006-291x(67)90526-8. [DOI] [PubMed] [Google Scholar]
- Rega A. F., Pouchan M. I., Garrahan P. J. Potassium ions asymmetrically activate erythrocyte membrane phosphatase. Science. 1970 Jan 2;167(3914):55–56. doi: 10.1126/science.167.3914.55. [DOI] [PubMed] [Google Scholar]
- Sachs J. R. Ouabain-insensitive sodium movements in the human red blood cell. J Gen Physiol. 1971 Mar;57(3):259–282. doi: 10.1085/jgp.57.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs J. R. Sodium movements in the human red blood cell. J Gen Physiol. 1970 Sep;56(3):322–341. doi: 10.1085/jgp.56.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uesugi S., Dulak N. C., Dixon J. F., Hexum T. D., Dahl J. L., Perdue J. F., Hokin L. E. Studies on the characterization of the sodium-potassium transport adenosine triphosphatase. VI. Large scale partial purification and properties of a lubrol-solubilized bovine brain enzyme. J Biol Chem. 1971 Jan 25;246(2):531–543. [PubMed] [Google Scholar]


