Abstract
Objective
To investigate oxidative stress biomarkers in a cross-sectional pilot study of 50 participants with sporadic ALS (sALS) compared to 46 control subjects.
Methods
We measured urinary 8-oxodeoxyguanosine (8-oxodG), urinary 15-F2t-isoprostane (IsoP), and plasma protein carbonyl by ELISA methods. We also determined if ELISA measurement of 8-oxodG could be validated against measures from high pressure liquid chromatography coupled with electrochemical detection, the current standard method.
Results
8-oxodG and IsoP levels adjusted for creatinine were significantly elevated in sALS participants. These differences persisted after age and gender were controlled in regression analyses. These markers are highly and positively correlated with each other. 8-oxodG measured by the two techniques from the same urine sample were positively correlated (P < .0001). Protein carbonyl was not different between sALS participants and controls.
Conclusion
Using ELISA we confirmed that certain oxidative stress biomarkers were elevated in sALS participants. ELISA may be reliable and thus useful in epidemiology studies requiring large numbers of samples to determine the significance of increased oxidative stress markers in sALS. Further studies are required.
Search Terms: epidemiology, amyotrophic lateral sclerosis (ALS), biomarkers, oxidative stress, neurodegeneration
INTRODUCTION
Despite decades of intense research efforts and a number of highly plausible hypotheses, the cause of sporadic ALS (sALS) has not been determined [1]. Factors related to the risk of developing sALS have only recently been extensively investigated. It is considered a multifactorial and complex disease, that is, genetic, environmental, or genetic-environmental interactions may lead to motor neuronal degeneration [2, 3]. Oxidative stress, a condition whereby the pro- and anti-oxidant balance is disrupted, increases reactive oxygen and nitrogen species (ROS, RNS), which damage cellular constituents of motor neurons and surrounding cells. Oxidative stress has been closely associated with motor neuron degeneration in sALS [3–5].
Several recent clinical studies support an oxidative stress hypothesis as a number of biomarkers for oxidative stress have been found abnormal in ALS [6–11]. In particular, Bogdanov et al.. [6] showed that in 65 participants newly diagnosed with sALS, 63 healthy participants, and 37 participants with non-ALS neurological disorders, 8-oxo (or 8-hydroxy) deoxyguanosine (8-oxodG), a marker of DNA damage and repair, was elevated in cerebral spinal fluid (CSF), plasma and urine only in sALS participants [6]. In all participants, plasma and CSF 8-oxodG levels increased with age, providing evidence, as expected, that oxidative damage is associated with normal aging. In another study, the lipid peroxidation product 4-hydroxy-2,3-nonenal was elevated in CSF and serum in participants with sALS compared to normal controls [7]. These studies suggest that certain blood and urinary biomarkers might be used to study oxidative stress in patients with sALS.
A number of epidemiological studies conducted in participants with sALS suggest that diverse environmental and lifestyle factors are associated with the occurrence of sALS. Many of these purported risk factors may generate systemic oxidative stress (see Discussion below). To explore possible associations between these environmental/lifestyle factors, oxidative stress and sALS, a well-designed molecular epidemiological study is necessary. For such a study, efficient and reliable measurement techniques for oxidative stress biomarkers are crucial. Therefore, in a pilot study we analyzed whether these biomarkers differ significantly between participants with sALS and healthy participants.
We chose the three most frequently utilized and easily accessible oxidative stress biomarkers [12]. 8-oxodG is one of the principal DNA adducts, derived from mitochondrial DNA and nuclear DNA [13, 14]. 15-F2t-isoprostane (IsoP) is derived from arachidonic acid via a free radical-catalyzed mechanism. It is used extensively as a clinical biomarker in various diseases including Alzheimer’s disease [15, 16]. DNA adducts and lipid peroxidation products indicate current oxidative stress, while plasma protein carbonyl, an end product of intracellular amino acids damaged by excessive ROS, indicates longer term (approximately 3 months) oxidative stress [17].
SUBJECTS, MATERIALS AND METHODS
Study Subjects
After receiving Institutional Review Board and HIPAA approvals at Columbia University Medical Center, we collected plasma and urine samples from volunteers: 50 participants with sporadic ALS (sALS) and 46 controls at the Eleanor and Lou Gehrig MDA/ALS Research Center. The diagnosis of sALS was made based on El Escorial ALS Diagnostic Criteria [18] and included definite, probable, laboratory-supported probable and possible ALS. The diagnosis of sALS was primarily based on no family history of ALS and, for a very few participants, SOD1 molecular testing with negative results. Spouses, siblings, children and friends of sALS participants were asked to volunteer as controls if they were free of neurodegenerative disease and clinically healthy without any specific selection scheme. This pilot study was cross-sectional in design. There was no restriction as to food and medication intake prior to specimen collection, and all specimens were obtained during the daytime.
Demographic data including age and gender were obtained for all subjects. In the participants with sALS, disease duration (time from clinically recognizable symptom onset to examination date) was ascertained, and ALSFRS-R [19] and FVC were measured.
Biomarker Measurements
All assays were conducted with laboratories blinded to status as a participant with sALS or a control.
Urinary 8-oxodG levels were determined by competitive ELISA essentially as described previously [20]. Briefly, wells were coated with 8-oxoG conjugated with BSA. 8-oxodG standards (concentration range 5 – 80 ng/ml) and urine samples (diluted 1:1 with PBS) were assayed with antibody 1F7 [20]. Incubation with secondary antibody conjugated with alkaline phosphatase was followed by incubation with p-nitrophenyl phosphate.
Urinary 8-oxodG was also determined by high pressure liquid chromatography (HPLC) and electrochemical detection (ECD) as previously described [21]. Briefly, the method is based on the unique purine selectivity of porous carbon columns which allows routine accurate measurement of 8-oxodG in a variety of biological matrices. Samples are injected onto a C8 column, and the band containing 8-oxodG is then quantitatively trapped on a carbon column. The selectivity of the carbon column for 8-oxodG allows elimination of interfering peaks by washing the column with a second mobile phase, followed by elution of 8-oxodG to a C18 column with an identical mobile phase containing adenosine to displace 8-oxodG. Detection with series coulometric electrodes provides 0.5 pg sensitivity for 8-oxodG and qualitative certainty by response ratios. Because urinary 8-oxodG concentration depends on individual urinary output, it was normalized using urinary creatinine levels, assayed using a kit from Sigma (St. Louis, MO; Cat. No. 555-A) [22].
Urinary 15-F2t-isoprostane (IsoP) was analyzed using immunoassay kits from Oxford Biomedical Research (Oxford, MI; Product No. EA 85) according to the manufacturer’s recommendations. As 8-oxodG, urinary IsoP was also normalized using urinary creatinine levels as described above.
Plasma protein carbonyl
Plasma oxidized protein was measured as follows: The concentration of total plasma proteins was determined using the BioRad BCA assay in 96-well plates. Plasma was diluted to 4 mg/ml and derivatization by dinitrophenyl hydrazine was carried out essentially as described [23]. A standard curve for the noncompetitive ELISA was generated by mixing sodium borohydride-reduced and HOCl-oxidized bovine serum albumin (BSA), prepared as described [23]. Carbonyl concentration in the BSA standard was calculated colorimetrically (A375nm). Standard protein solutions were derivatized with DNPH then adsorbed into the wells of an ELISA plate as for the test samples, before incubating with biotin conjugated anti-DNPH antibody (Molecular Probes, Eugene, OR). The biotin-conjugated primary antibody was then detected with streptavidin-biotinylated horseradish peroxidase.
Statistical Analyses
Variables with skewed distributions were appropriately transformed to reduce the impact of extreme values in the statistical analysis. Since some variables had skewed distributions and were dissimilar within group variances, we used the non-parametric Wilcoxon’s rank-sum test to assess differences between subjects with ALS and controls. Patient-control differences in categorical variables were assessed using Chi-square tests. Additionally, we estimated correlations between continuous variables using Spearman correlation methods. Finally, we estimated the associations between biomarkers of oxidative stress and sALS using logistic regression, controlling for age and sex.
RESULTS
Table 1 summarizes the clinical features of participants with sALS and controls, along with the results of the oxidative marker measurements. Cases were more likely to be male, and were older than controls. Urinary IsoP and urinary 8-oxodG were higher among cases than among controls, before adjustment for urine concentration (urinary creatinine). As expected [24, 25], 8-oxodG (adjusted for creatinine) increased with age (r=0.30, p=.004). No relationship was found between age and urinary IsoP (adjusted for creatinine). Plasma protein carbonyl levels were not different between patients and controls before and after adjustment for age
Table 1.
8-oxodG, IsoP and Protein Carbonyl, and Disease Status Measures in sporadic ALS Patients and Controls
| Variable | ALS Subjects (n=50*) | Control subjects (n=46*) | p-value* | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Mean | S.D. | Range | Mean | S.D. | Range | ||
| Male (%) | 61 | --- | --- | 33 | --- | --- | .009 |
| Age (years) | 61 | 12 | 23, 80 | 51 | 14 | 22,81 | .050 |
| ALSFRS-R score | 31 | 9 | 8, 46 | --- | --- | --- | --- |
| FVC (% of predicted) | 77 | 23 | 16, 120 | --- | --- | --- | --- |
| Disease duration (years) | 3 | 2 | 1, 13 | --- | --- | --- | --- |
| Urinary Isoprostane** (nmol/L) | 7 | 6 | 0.3, 24 | 5 | 5 | 0.4, 22 | .035 |
| 8-oxodG** (nmol/L) | 415 | 214 | 31,1301 | 362 | 304 | 77, 1665 | .108 |
| Plasma Protein carbonyl** (nmol/ml) | 24 | 12 | 6, 59 | 22 | 9 | 9, 51 | .733 |
| Creatinine (mmol/L) | 9 | 6 | 1, 30 | 9 | 6 | 2, 27 | .780 |
| Isoprostane/Creatinine** (nmol/mmol) | 0.8 | 0.8 | 0.3, 5.0 | 0.5 | 0.2 | 0.2, 1.1 | .002 |
| 8-oxodG/Creatinine** (nmol/mmol) | 57 | 38 | 16, 203 | 42 | 19 | 12, 122 | .036 |
While n=50 for ALS subjects and n=46 for controls in total, there were a limited number of missing data points for some variables because biological samples were unavailable or not usable or clinical data were not obtained. For any given variable among cases, the lowest N is equal to 42 and for controls, the lowest N is equal to 41.
Each measured by ELISA methods.
Urinary 8-oxodG levels (adjusted for creatinine) were significantly higher in sALS participants than in controls (p=.036). Data distribution for cases and controls is shown in Figure 1a. Using logistic regression analysis controlling for sex and age, we found a positive association between sALS and the natural log of urinary 8-oxodG (adjusted for creatinine). The odds ratio (95% CI; p value) was 3.01 (1.26, 7.14; .001) for doubling the exposure level of urinary 8-oxodG (adjusted for creatinine).
Figure 1.
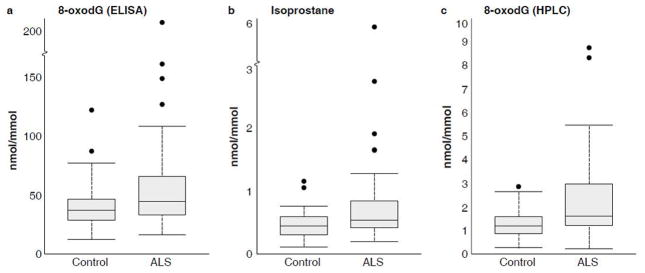
Data distributions for urinary oxidative stress markers (adjusted for creatinine): a) 8-oxodG measured by ELISA, b) isoprostane measured by ELISA, and c) 8-oxodG measured by HPLC/ECD. For each boxplot, the middle dark line represents the median. The shaded box represents the middle two quartiles (Q2 and Q3) of the data. The brackets at the end of the dashed lines represent the data point closest to and within 150% of the Q2/Q3 data span. The dots represent outlier values.
Urinary IsoP levels (adjusted for creatinine) were significantly higher in sALS participants than in controls (p = .002). Data distribution for cases and controls is shown in Figure 1b. Using logistic regression controlling for sex and age, we found a positive association between sALS and the natural log of urinary IsoP (adjusted for creatinine). The odds ratio (95% CI; p value) was 3.24 (1.40, 7.55; .006) for doubling the exposure level of urinary IsoP (adjusted for creatinine).
Levels of 8-oxodG measured by ELISA and HPLC/ECD using the same urine specimens were correlated although values measured by ELISA were higher than those measured by HPLC/ECD [21]. The Spearman correlation coefficient was .545 (p < .0001) (Figure 2).
Figure 2.
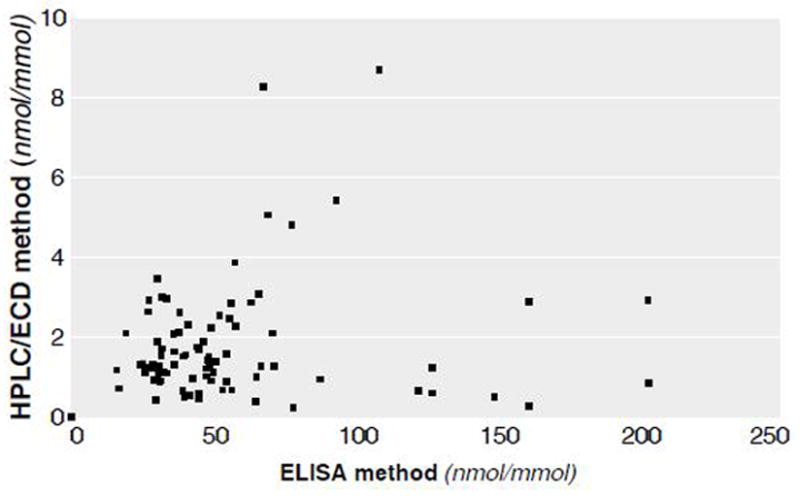
Correlation of 8-oxodG, measured by ELISA and by high pressure liquid chromatography coupled with electrochemical detector (HPLC/ECD) in the same urine samples.
Figure 1c shows the data distribution for urinary 8-oxodG levels (adjusted for creatinine) as measured by HPLC/ECD. These levels were significantly higher in sALS participants than in controls (p = .004). Using logistic regression controlling for sex and age, we found a positive association between sALS and the natural log of urinary 8-oxodG (adjusted for creatinine). The odds ratio (95% CI; p value) was 2.02 (1.16, 3.51; p = .012) for doubling the exposure level.
Correlation among oxidative markers
As expected, 8-oxodG and IsoP had a significant positive Spearman correlation (r = .70, p < .001). 8-oxodG measured by HPLC also had a significant Spearman correlation with isoprostane (r = .60, p < .001). There was no significant Spearman correlation between either of these two markers and plasma protein carbonyl.
Correlation between OS biomarkers and clinical measures
No significant Spearman correlations were found between both 8-oxodG and IsoP (both adjusted for creatinine) and the clinical measures of ALSFRS-r and FVC. Repeated measures of ALSFRS-r and FVC prior to and including the date of urine collection were available in 20 patients with ALS. The trend in these clinical measures was not significantly associated with the 8-oxodG and IsoP (adjusted for creatinine).
DISCUSSION
Two out of the three oxidative stress biomarkers measured in this cross-sectional study, the oxidized DNA adduct and the lipid peroxidation product, were significantly increased in sALS participants compared to controls. In addition, the correlation coefficient between the ELISA and HPLC/ECD methods in measuring 8-oxodG of the same urine specimen was 0.545 (p < .0001), which was significant; however for the definitive reliability study a higher coefficient is desirable. The ELISA values were usually 10–20-fold higher than those by HPLC. While the HPLC method measures a single compound, the ELISA method can detect 8-oxoguanine, 8-oxoG and 8-oxodG as well as oligonucleotides containing 8-oxodG. There is also some cross reactivity with guanine and structurally related derivatives such as 8-mercaptoguanosine [20]. A previous study comparing HPLC and ELISA methods measuring 8-oxodG, found the ELISA method more reliable when repeated measures were used [26]. When using ELISA techniques, additional care must be taken in order to reduce variability, such as collecting the urine specimen in the morning after fasting and before any medications are taken.
In contrast to urinary 8-oxodG and IsoP, protein carbonyl levels did not differ between participants with sALS and controls. 8-oxodG and IsoP were strongly correlated with each other but not with protein carbonyl, indicating that the oxidative biomarkers we measured are measuring different aspects of oxidative stress. Therefore, it is important to analyze several biomarkers when studying oxidative stress in sALS
If oxidative stress markers are increased in sALS, what is the biological mechanism underlying this increase? A previous study of 8-oxodG in CSF, plasma and urine in patients with ALS found that the plasma levels of DNA adduct were elevated far more than expected if CSF was the sole source of elevated oxidative stress marker levels, suggesting that increased oxidative stress found in blood and urine are likely derived from outside of the CNS [6]. In fact, a recent series of studies suggest the possibility of systemic involvement in sALS [27]. In ALS patients, cultured monocytes have increased inflammatory cytokine production [28], cutaneous collagen fibers are abnormal [29], systemic glutamate metabolism is impaired [30], the antioxidative defense system of erythrocytes is reduced [31], patients are in a hypermetabolic state due to abnormal skeletal muscle metabolism [32], and muscle mitochondria are not only structurally abnormal [33,34], but have primary abnormalities known to cause an ALS-like syndrome [35]. A major source of ‘systemic’ oxidative stress is clearly generated in skeletal muscle, which constitutes 40 to 45% of body mass [36]. All these reports thus suggest that sALS may have increased oxidative stress in the system outside of central nervous system.
Another crucial question regarding increased oxidative stress in sALS is specificity. Recent studies have suggested that abnormal oxidative stress may be a common process in a number of neurodegenerative diseases, such as Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease [37–41] and in a number of severe chronic systemic diseases [36]. The latter examples include advanced cancer [42], severe chronic obstructive pulmonary disease [43], renal failure [44], and HIV, all of which cause development of cachexia in relation to underlying oxidative stress. These observations suggest that oxidative stress may be a common path leading to severe chronic systemic diseases [36]. Cachexia in ALS is in fact a unique problem first pointed out by Norris [45], in which severe cachexia may develop without much muscle atrophy in some patients [46]. However, we must investigate in a prospective study whether increased oxidative stress has any causal relationship to progressive weight loss in ALS.
There are a number of factors that have been associated with ALS [47]. Most factors listed there are known to cause systemic oxidative stress in in-vivo and in-vitro studies, including a number of agricultural chemicals [48–50]; lead and other heavy metals [51–54] and electromagnetic fields [55]. Specific lifestyle factors such as excessive physical exertion, smoking, consumption of glutamate-rich and high-fat foods are also shown to generate systemic oxidative stress [50, 56–58]. The factors involved in military service are complex, but such activities may similarly cause significant oxidative stress [59–61]. The relationship between environmental and lifestyle factors and increased oxidative stress in patients with sALS must be established more clearly.
Our study had a number of limitations primarily because it was designed as cross-sectional pilot study. For example, only a small number of sALS participants had clinical measures that could be evaluated with oxidative marker measures. There were also large overlaps in the levels of oxidative stress markers between participants with sALS and controls. Although results clearly suggest underlying pathomechanisms, these markers cannot be used for clinical diagnostic purposes. In this pilot study, urine and blood samples were obtained whenever available from the subjects. This may be partly responsible for the differences in 8-oxodG levels by ELISA and HPLC. The ELISA is known to cross react with structurally similar compounds as discussed earlier. For future studies, we will need to strictly control the timing of specimen sampling, fasting status, and medication status upon blood and urine sampling. Another limitation was the selection of controls, which were not age and gender matched although these variables were controlled in a logistic regression analysis. Matched controls are preferred for future studies to ensure adequate control for potentially confounding variables.
This study demonstrated that urine, which is simple and convenient to collect, can be useful in biomarker studies. In addition, the ELISA method for 8-oxodG and IsoP measurements was found to be reliable at least at one point in time This is important because ELISA is more suitable for a study with a large sample size because it is less time-consuming and expensive than the HPLC,. This pilot study provides a strong evidence that a molecular epidemiological study using ELISA methods to measure oxidative stress markers is feasible.
Acknowledgments
This study was funded by the Center for Environmental Health in Northern Manhattan (P30ES009089—Regina Santella, PI), the Muscular Dystrophy Association, the Wings Over Wall Street Fund, the Ride for Life, the Spina Golf for Life, the Adams Foundation and by the generous donations of individuals. Linda Ali Cruz, MA coordinated the initial part of the study. Jonathan Sandner, BA assisted with additional data collection. We thank the volunteers, their families, and their friends for participating in this study.
Footnotes
Conflict of Interest Statement: None of the authors have conflicts of interests in relation to the presented study. HM received a research grant from Aeolus, served as a consultant for Eisai Pharma, received an honorarium for an advisory board meeting for Teva, and received educational grants for a CME course at Columbia from Sanofi-Aventis, Avenia, Ethena, and Bioscrips.
References
- 1.Rowland LP. The cause of sporadic ALS. In: Mitsumoto HPS, Gordon PH, editors. Amyotrophic Lateral Sclerosis. New York: Taylor and Francis; 2006. pp. 81–98. [Google Scholar]
- 2.Bruijn LI, Miller TM, Cleveland DW. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu Rev Neurosci. 2004;27:723–49. doi: 10.1146/annurev.neuro.27.070203.144244. [DOI] [PubMed] [Google Scholar]
- 3.Shaw PJ. Molecular and cellular pathways of neurodegeneration in motor neurone disease. J Neurol Neurosurg Psychiatry. 2005 Aug;76(8):1046–57. doi: 10.1136/jnnp.2004.048652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersen JK. Oxidative stress in neurodegeneration: cause or consequence? Nat Med. 2004 Jul;10( Suppl):S18–25. doi: 10.1038/nrn1434. [DOI] [PubMed] [Google Scholar]
- 5.Beckman JSEA. Superoxide dismutase, oxidative stress, and ALS. In: Mitsumoto H, Perzedborski S, Gordon PH, editors. Amyotrophic Lateral Sclerosis. New York: Taylor and Francis; 2005. pp. 339–54. [Google Scholar]
- 6.Bogdanov M, Brown RH, Matson W, Smart R, Hayden D, O’Donnell H, et al. Increased oxidative damage to DNA in ALS patients. Free Radic Biol Med. 2000 Oct 1;29(7):652–8. doi: 10.1016/s0891-5849(00)00349-x. [DOI] [PubMed] [Google Scholar]
- 7.Simpson EP, Henry YK, Henkel JS, Smith RG, Appel SH. Increased lipid peroxidation in sera of ALS patients: a potential biomarker of disease burden. Neurology. 2004 May 25;62(10):1758–65. doi: 10.1212/wnl.62.10.1758. [DOI] [PubMed] [Google Scholar]
- 8.Yoshino H, Kimura A. Investigation of the therapeutic effects of edaravone, a free radical scavenger, on amyotrophic lateral sclerosis (Phase II study) Amyotroph Lateral Scler. 2006 Dec;7(4):241–5. doi: 10.1080/17482960600881870. [DOI] [PubMed] [Google Scholar]
- 9.Bonnefont-Rousselot D, Lacomblez L, Jaudon M, Lepage S, Salachas F, Bensimon G, et al. Blood oxidative stress in amyotrophic lateral sclerosis. J Neurol Sci. 2000 Sep 1;178(1):57–62. doi: 10.1016/s0022-510x(00)00365-8. [DOI] [PubMed] [Google Scholar]
- 10.Aoyama K, Matsubara K, Fujikawa Y, Nagahiro Y, Shimizu K, Umegae N, et al. Nitration of manganese superoxide dismutase in cerebrospinal fluids is a marker for peroxynitrite-mediated oxidative stress in neurodegenerative diseases. Ann Neurol. 2000 Apr;47(4):524–7. [PubMed] [Google Scholar]
- 11.Kaufmann E, Boehm BO, Sussmuth SD, Kientsch-Engel R, Sperfeld A, Ludolph AC, et al. The advanced glycation end-product N epsilon-(carboxymethyl)lysine level is elevated in cerebrospinal fluid of patients with amyotrophic lateral sclerosis. Neurosci Lett. 2004 Nov 23;371(2–3):226–9. doi: 10.1016/j.neulet.2004.08.071. [DOI] [PubMed] [Google Scholar]
- 12.Mariani E, Polidori MC, Cherubini A, Mecocci P. Oxidative stress in brain aging, neurodegenerative and vascular diseases: an overview. J Chromatogr B Analyt Technol Biomed Life Sci. 2005 Nov 15;827(1):65–75. doi: 10.1016/j.jchromb.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 13.Beckman KB, Ames BN. Endogenous oxidative damage of mtDNA. Mutation research. 1999 Mar 8;424(1–2):51–8. doi: 10.1016/s0027-5107(99)00007-x. [DOI] [PubMed] [Google Scholar]
- 14.de Souza-Pinto NC, Eide L, Hogue BA, Thybo T, Stevnsner T, Seeberg E, et al. Repair of 8-oxodeoxyguanosine lesions in mitochondrial dna depends on the oxoguanine dna glycosylase (OGG1) gene and 8-oxoguanine accumulates in the mitochondrial dna of OGG1-defective mice. Cancer research. 2001 Jul 15;61(14):5378–81. [PubMed] [Google Scholar]
- 15.Montuschi P, Barnes PJ, Roberts LJ., 2nd Isoprostanes: markers and mediators of oxidative stress. Faseb J. 2004 Dec;18(15):1791–800. doi: 10.1096/fj.04-2330rev. [DOI] [PubMed] [Google Scholar]
- 16.Rossner P, Jr, Gammon MD, Terry MB, Agrawal M, Zhang FF, Teitelbaum SL, et al. Relationship between urinary 15-F2t-isoprostane and 8-oxodeoxyguanosine levels and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2006 Apr;15(4):639–44. doi: 10.1158/1055-9965.EPI-05-0554. [DOI] [PubMed] [Google Scholar]
- 17.Dalle-Donne I, Aldini G, Carini M, Colombo R, Rossi R, Milzani A. Protein carbonylation, cellular dysfunction, and disease progression. J Cell Mol Med. 2006 Apr-Jun;10(2):389–406. doi: 10.1111/j.1582-4934.2006.tb00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Federation NRGoAS. A revised El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. 2000 doi: 10.1080/146608200300079536. [cited; Available from: www.wfnals.org. [DOI] [PubMed]
- 19.Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III) J Neurol Sci. 1999 Oct 31;169(1–2):13–21. doi: 10.1016/s0022-510x(99)00210-5. [DOI] [PubMed] [Google Scholar]
- 20.Yin B, Whyatt RM, Perera FP, Randall MC, Cooper TB, Santella RM. Determination of 8-hydroxydeoxyguanosine by an immunoaffinity chromatography-monoclonal antibody-based ELISA. Free Radic Biol Med. 1995 Jun;18(6):1023–32. doi: 10.1016/0891-5849(95)00003-g. [DOI] [PubMed] [Google Scholar]
- 21.Bogdanov MB, Beal MF, McCabe DR, Griffin RM, Matson WR. A carbon column-based liquid chromatography electrochemical approach to routine 8-hydroxy-2′-deoxyguanosine measurements in urine and other biologic matrices: a one-year evaluation of methods. Free Radic Biol Med. 1999 Sep;27(5–6):647–66. doi: 10.1016/s0891-5849(99)00113-6. [DOI] [PubMed] [Google Scholar]
- 22.Morrow JD. The isoprostanes: their quantification as an index of oxidant stress status in vivo. Drug Metab Rev. 2000 Aug-Nov;32(3–4):377–85. doi: 10.1081/dmr-100102340. [DOI] [PubMed] [Google Scholar]
- 23.Buss H, Chan TP, Sluis KB, Domigan NM, Winterbourn CC. Protein carbonyl measurement by a sensitive ELISA method. Free Radic Biol Med. 1997;23(3):361–6. doi: 10.1016/s0891-5849(97)00104-4. [DOI] [PubMed] [Google Scholar]
- 24.Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, et al. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci U S A. 2005 Apr 12;102(15):5618–23. doi: 10.1073/pnas.0501559102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ward WF, Qi W, Van Remmen H, Zackert WE, Roberts LJ, 2nd, Richardson A. Effects of age and caloric restriction on lipid peroxidation: measurement of oxidative stress by F2-isoprostane levels. J Gerontol A Biol Sci Med Sci. 2005 Jul;60(7):847–51. doi: 10.1093/gerona/60.7.847. [DOI] [PubMed] [Google Scholar]
- 26.Shimoi K, Kasai H, Yokota N, Toyokuni S, Kinae N. Comparison between high-performance liquid chromatography and enzyme-linked immunosorbent assay for the determination of 8-hydroxy-2′-deoxyguanosine in human urine. Cancer Epidemiol Biomarkers Prev. 2002 Aug;11(8):767–70. [PubMed] [Google Scholar]
- 27.Appel SH. Is ALS a systemic disorder? Evidence from muscle mitochondria. Exp Neurol. 2006 Mar;198(1):1–3. doi: 10.1016/j.expneurol.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 28.Lancero HL, Do H, Gascon R, et al. Observed elevated cytokines and myeloid/interferon-induced (MIFN) signature protein levels in ALS: a possible ALS early detection method. Amyotroph Lateral Scler. 2006;7(suppl 1 ):63–6. [Google Scholar]
- 29.Ono S. The skin in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000 Jun;1(3):191–9. doi: 10.1080/14660820050515188. [DOI] [PubMed] [Google Scholar]
- 30.Plaitakis A, Caroscio JT. Abnormal glutamate metabolism in amyotrophic lateral sclerosis. Ann Neurol. 1987 Nov;22(5):575–9. doi: 10.1002/ana.410220503. [DOI] [PubMed] [Google Scholar]
- 31.Nikolic-Kokic A, Stevic Z, Blagojevic D, Davidovic B, Jones DR, Spasic MB. Alterations in anti-oxidative defence enzymes in erythrocytes from sporadic amyotrophic lateral sclerosis (SALS) and familial ALS patients. Clin Chem Lab Med. 2006;44(5):589–93. doi: 10.1515/CCLM.2006.111. [DOI] [PubMed] [Google Scholar]
- 32.Desport JC, Torny F, Lacoste M, Preux PM, Couratier P. Hypermetabolism in ALS: correlations with clinical and paraclinical parameters. Neurodegener Dis. 2005;2(3–4):202–7. doi: 10.1159/000089626. [DOI] [PubMed] [Google Scholar]
- 33.Dupuis L, Gonzalez de Aguilar JL, Oudart H, de Tapia M, Barbeito L, Loeffler JP. Mitochondria in amyotrophic lateral sclerosis: a trigger and a target. Neurodegener Dis. 2004;1(6):245–54. doi: 10.1159/000085063. [DOI] [PubMed] [Google Scholar]
- 34.Sasaki S, Iwata M. Mitochondrial alterations in the spinal cord of patients with sporadic amyotrophic lateral sclerosis. J Neuropathol Exp Neurol. 2007 Jan;66(1):10–6. doi: 10.1097/nen.0b013e31802c396b. [DOI] [PubMed] [Google Scholar]
- 35.Finsterer J. Mitochondriopathy mimicking amyotrophic lateral sclerosis. Neurologist. 2003 Jan;9(1):45–8. doi: 10.1097/01.nrl.0000038589.58012.a8. [DOI] [PubMed] [Google Scholar]
- 36.Moylan JS, Reid MB. Oxidative stress, chronic disease, and muscle wasting. Muscle Nerve. 2007 Jan 31;35(4):411–29. doi: 10.1002/mus.20743. [DOI] [PubMed] [Google Scholar]
- 37.Casadesus G, Smith MA, Basu S, Hua J, Capobianco DE, Siedlak SL, et al. Increased isoprostane and prostaglandin are prominent in neurons in Alzheimer disease. Molecular neurodegeneration. 2007;2:2. doi: 10.1186/1750-1326-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hersch SM, Gevorkian S, Marder K, Moskowitz C, Feigin A, Cox M, et al. Creatine in Huntington disease is safe, tolerable, bioavailable in brain and reduces serum 8OH2’dG. Neurology. 2006 Jan 24;66(2):250–2. doi: 10.1212/01.wnl.0000194318.74946.b6. [DOI] [PubMed] [Google Scholar]
- 39.Kikuchi A, Takeda A, Onodera H, Kimpara T, Hisanaga K, Sato N, et al. Systemic increase of oxidative nucleic acid damage in Parkinson’s disease and multiple system atrophy. Neurobiol Dis. 2002 Mar;9(2):244–8. doi: 10.1006/nbdi.2002.0466. [DOI] [PubMed] [Google Scholar]
- 40.Montine TJ, Beal MF, Cudkowicz ME, O’Donnell H, Margolin RA, McFarland L, et al. Increased CSF F2-isoprostane concentration in probable AD. Neurology. 1999 Feb;52(3):562–5. doi: 10.1212/wnl.52.3.562. [DOI] [PubMed] [Google Scholar]
- 41.Sato S, Mizuno Y, Hattori N. Urinary 8-hydroxydeoxyguanosine levels as a biomarker for progression of Parkinson disease. Neurology. 2005 Mar 22;64(6):1081–3. doi: 10.1212/01.WNL.0000154597.24838.6B. [DOI] [PubMed] [Google Scholar]
- 42.Mantovani G, Maccio A, Madeddu C, Massa E. Cancer-related cachexia and oxidative stress: beyond current therapeutic options. Expert review of anticancer therapy. 2003 Jun;3(3):381–92. doi: 10.1586/14737140.3.3.381. [DOI] [PubMed] [Google Scholar]
- 43.Balasubramanian VP, Varkey B. Chronic obstructive pulmonary disease: effects beyond the lungs. Current opinion in pulmonary medicine. 2006 Mar;12(2):106–12. doi: 10.1097/01.mcp.0000208449.73101.ac. [DOI] [PubMed] [Google Scholar]
- 44.Kalantar-Zadeh K, Brennan ML, Hazen SL. Serum myeloperoxidase and mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2006 Jul;48(1):59–68. doi: 10.1053/j.ajkd.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 45.Norris FH, Denys EH, UKS . Old and new clinical problems in amyotrophic lateral sclerosis. In: Tsubaki TTY, editor. Amyotrophic lateral sclerosis. Baltimore: University Park Press; 1978. pp. 3–26. [Google Scholar]
- 46.Desport JC, Preux PM, Truong TC, Vallat JM, Sautereau D, Couratier P. Nutritional status is a prognostic factor for survival in ALS patients. Neurology. 1999 Sep 22;53(5):1059–63. doi: 10.1212/wnl.53.5.1059. [DOI] [PubMed] [Google Scholar]
- 47.McGuire V, Nelson LM. Epidemiology of ALS. In: Mitsumoto HPS, Gordon PH, editors. Amyotrophic Lateral Sclerosis. New York: Taylor and Francis; 2006. pp. 17–42. [Google Scholar]
- 48.Abou-Donia MB. Organophosphorus ester-induced chronic neurotoxicity. Arch Environ Health. 2003 Aug;58(8):484–97. doi: 10.3200/AEOH.58.8.484-497. [DOI] [PubMed] [Google Scholar]
- 49.Kovacic P. Mechanism of organophosphates (nerve gases and pesticides) and antidotes: electron transfer and oxidative stress. Curr Med Chem. 2003 Dec;10(24):2705–9. doi: 10.2174/0929867033456314. [DOI] [PubMed] [Google Scholar]
- 50.Luo J, Shi R. Acrolein induces axolemmal disruption, oxidative stress, and mitochondrial impairment in spinal cord tissue. Neurochem Int. 2004 Jun;44(7):475–86. doi: 10.1016/j.neuint.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 51.Schmuck G, Rohrdanz E, Tran-Thi QH, Kahl R, Schluter G. Oxidative stress in rat cortical neurons and astrocytes induced by paraquat in vitro. Neurotox Res. 2002 Feb;4(1):1–13. doi: 10.1080/10298420290007574. [DOI] [PubMed] [Google Scholar]
- 52.Chetty CS, Vemuri MC, Campbell K, Suresh C. Lead-induced cell death of human neuroblastoma cells involves GSH deprivation. Cell Mol Biol Lett. 2005;10(3):413–23. [PubMed] [Google Scholar]
- 53.Fowler BA, Whittaker MH, Lipsky M, Wang G, Chen XQ. Oxidative stress induced by lead, cadmium and arsenic mixtures: 30-day, 90-day, and 180-day drinking water studies in rats: an overview. Biometals. 2004 Oct;17(5):567–8. doi: 10.1023/b:biom.0000045740.52182.9d. [DOI] [PubMed] [Google Scholar]
- 54.Qian Y, Zheng Y, Ramos KS, Tiffany-Castiglioni E. The involvement of copper transporter in lead-induced oxidative stress in astroglia. Neurochem Res. 2005 Apr;30(4):429–38. doi: 10.1007/s11064-005-2677-1. [DOI] [PubMed] [Google Scholar]
- 55.Lai H, Singh NP. Magnetic-field-induced DNA strand breaks in brain cells of the rat. Environ Health Perspect. 2004 May;112(6):687–94. doi: 10.1289/ehp.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nelson LM, McGuire V, Longstreth WT, Jr, Matkin C. Population-based case-control study of amyotrophic lateral sclerosis in western Washington State. I. Cigarette smoking and alcohol consumption. Am J Epidemiol. 2000 Jan 15;151(2):156–63. doi: 10.1093/oxfordjournals.aje.a010183. [DOI] [PubMed] [Google Scholar]
- 57.Vayssier M, Banzet N, Francois D, Bellmann K, Polla BS. Tobacco smoke induces both apoptosis and necrosis in mammalian cells: differential effects of HSP70. Am J Physiol. 1998 Oct;275(4 Pt 1):L771–9. doi: 10.1152/ajplung.1998.275.4.L771. [DOI] [PubMed] [Google Scholar]
- 58.Vollaard NB, Shearman JP, Cooper CE. Exercise-induced oxidative stress:myths, realities and physiological relevance. Sports Med. 2005;35(12):1045–62. doi: 10.2165/00007256-200535120-00004. [DOI] [PubMed] [Google Scholar]
- 59.Hayley S, Poulter MO, Merali Z, Anisman H. The pathogenesis of clinical depression: stressor- and cytokine-induced alterations of neuroplasticity. Neuroscience. 2005;135(3):659–78. doi: 10.1016/j.neuroscience.2005.03.051. [DOI] [PubMed] [Google Scholar]
- 60.Horner RD, Kamins KG, Feussner JR, Grambow SC, Hoff-Lindquist J, Harati Y, et al. Occurrence of amyotrophic lateral sclerosis among Gulf War veterans. Neurology. 2003 Sep 23;61(6):742–9. doi: 10.1212/01.wnl.0000069922.32557.ca. [DOI] [PubMed] [Google Scholar]
- 61.Weisskopf MG, O’Reilly EJ, McCullough ML, Calle EE, Thun MJ, Cudkowicz M, et al. Prospective study of military service and mortality from ALS. Neurology. 2005 Jan 11;64(1):32–7. doi: 10.1212/01.WNL.0000148649.17706.D9. [DOI] [PubMed] [Google Scholar]


