Abstract
A heterogeneous RNA fraction with properties resembling those of messenger RNA was identified in mammalian mitochondria. Synthesis of contaminating RNA of nuclear origin was suppressed by treatment with camptothecin. Labeling of the messenger-like RNA is completely inhibited by ethidium bromide, a specific inhibitor of mitochondrial functions.
Although mitochondrial protein synthesis resembles that of prokaryotes in several regards, the messenger-like RNA is covalently linked to poly(adenylic acid) [poly(A)]. Poly(A) has thus far been found only in eukaryotic cells. The poly(A) segment has a gel electrophoretic mobility of about 4 S, corresponding to a length of 50-80 nucleotides, and thus resembles in size the poly(A) found in some mammalian viral RNAs. The messenger RNA can be released from the mitochondrial protein-synthesizing structure by treatment with puromycin.
Keywords: camptothecin, poly(A), acrylamide gel electrophoresis
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelson H. T., Penman S. Selective interruption of high molecular weight RNA synthesis in HeLa cells by camptothecin. Nat New Biol. 1972 May 31;237(74):144–146. doi: 10.1038/newbio237144a0. [DOI] [PubMed] [Google Scholar]
- Armstrong J. A., Edmonds M., Nakazato H., Phillips B. A., Vaughn M. H. Polyadenylic acid sequences in the virion RNA of poliovirus and Eastern Equine Encephalitis virus. Science. 1972 May 5;176(4034):526–528. doi: 10.1126/science.176.4034.526. [DOI] [PubMed] [Google Scholar]
- Attardi B., Attardi G. Expression of the mitochondrial genome in HeLa cells. I. Properties of the discrete RNA components from the mitochondrial fraction. J Mol Biol. 1971 Jan 28;55(2):231–249. doi: 10.1016/0022-2836(71)90194-x. [DOI] [PubMed] [Google Scholar]
- Attardi G., Ojala D. Mitochondrial ribssome in HeLa cells. Nat New Biol. 1971 Feb 3;229(5):133–136. doi: 10.1038/newbio229133a0. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Wall R., Tushinski R. J. An adenylic acid-rich sequence in messenger RNA of HeLa cells and its possible relationship to reiterated sites in DNA. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1321–1325. doi: 10.1073/pnas.68.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Edmonds M., Vaughan M. H., Jr, Nakazato H. Polyadenylic acid sequences in the heterogeneous nuclear RNA and rapidly-labeled polyribosomal RNA of HeLa cells: possible evidence for a precursor relationship. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1336–1340. doi: 10.1073/pnas.68.6.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galper J. B., Darnell J. E. The presence of N-formyl-methionyl-tRNA in HeLa cell mitochondria. Biochem Biophys Res Commun. 1969 Jan 27;34(2):205–214. doi: 10.1016/0006-291x(69)90633-0. [DOI] [PubMed] [Google Scholar]
- Grollman A. P. Structural basis for inhibition of protein synthesis by emetine and cycloheximide based on an analogy between ipecac alkaloids and glutarimide antibiotics. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1867–1874. doi: 10.1073/pnas.56.6.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joklik W. K., Becker Y. Studies on the genesis of polyribosomes. I. Origin and significance of the subribosomal particles. J Mol Biol. 1965 Sep;13(2):496–510. doi: 10.1016/s0022-2836(65)80112-7. [DOI] [PubMed] [Google Scholar]
- Kessel D. Effects of camptothecin on RNA synthesis in leukemia L1210 cells. Biochim Biophys Acta. 1971 Aug 26;246(2):225–232. doi: 10.1016/0005-2787(71)90131-6. [DOI] [PubMed] [Google Scholar]
- Lee S. Y., Mendecki J., Brawerman G. A polynucleotide segment rich in adenylic acid in the rapidly-labeled polyribosomal RNA component of mouse sarcoma 180 ascites cells. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1331–1335. doi: 10.1073/pnas.68.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim L., Canellakis E. S. Adenine-rich polymer associated with rabbit reticulocyte messenger RNA. Nature. 1970 Aug 15;227(5259):710–712. doi: 10.1038/227710a0. [DOI] [PubMed] [Google Scholar]
- Penman S. RNA metabolism in the HeLa cell nucleus. J Mol Biol. 1966 May;17(1):117–130. doi: 10.1016/s0022-2836(66)80098-0. [DOI] [PubMed] [Google Scholar]
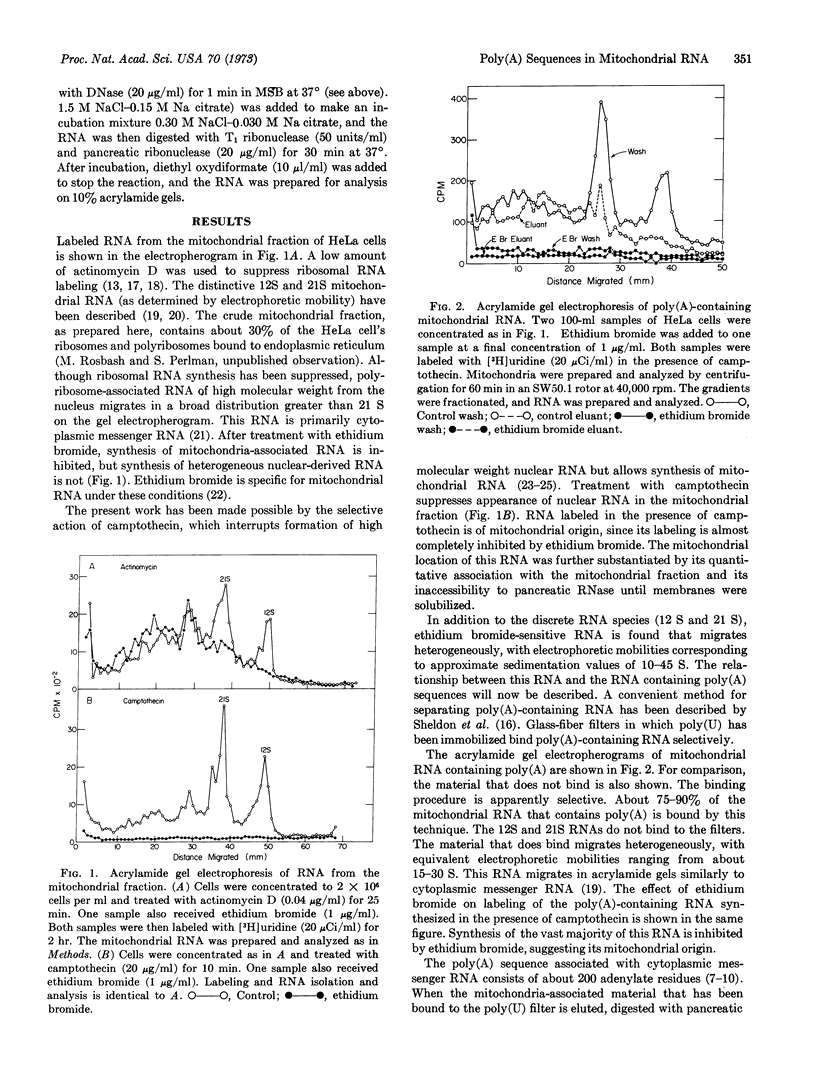
- Penman S., Vesco C., Penman M. Localization and kinetics of formation of nuclear heterodisperse RNA, cytoplasmic heterodisperse RNA and polyribosome-associated messenger RNA in HeLa cells. J Mol Biol. 1968 May 28;34(1):49–60. doi: 10.1016/0022-2836(68)90234-9. [DOI] [PubMed] [Google Scholar]
- Perlman S., Penman S. Mitochondrial protein synthesis: resistance to emetine and response to RNA synthesis inhibitors. Biochem Biophys Res Commun. 1970 Aug 24;40(4):941–948. doi: 10.1016/0006-291x(70)90994-0. [DOI] [PubMed] [Google Scholar]
- Perlman S., Penman S. Protein-synthesizing structures associated with mitochondria. Nature. 1970 Jul 11;227(5254):133–137. doi: 10.1038/227133a0. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E., LaTorre J. Lack of polyadenylic acid sequences in the messenger RNA of E. coli. Biochem Biophys Res Commun. 1972 Sep 26;48(6):1593–1600. doi: 10.1016/0006-291x(72)90896-0. [DOI] [PubMed] [Google Scholar]
- Roberts W. K., Newman J. F. Use of low concentrations of actinomycin D in the study of RNA synthesis in Ehrlich ascites cells. J Mol Biol. 1966 Sep;20(1):63–73. doi: 10.1016/0022-2836(66)90117-3. [DOI] [PubMed] [Google Scholar]
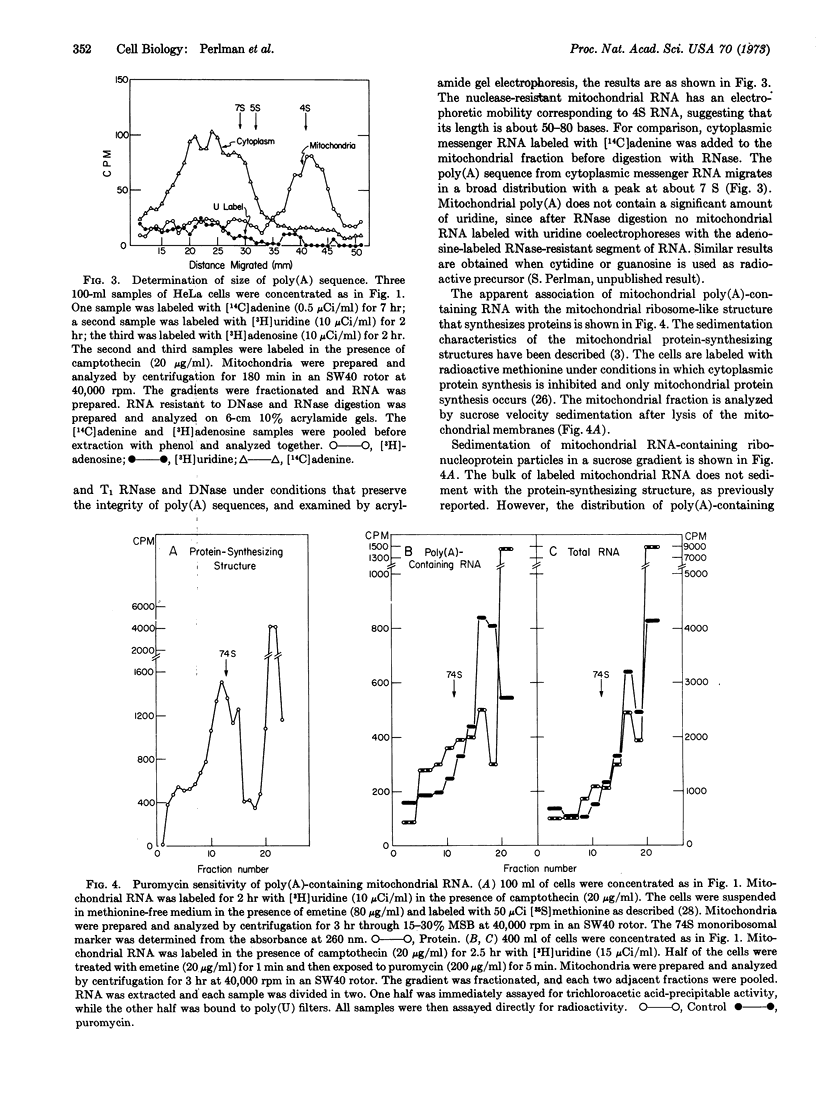
- Rosbash M., Penman S. Membrane-associated protein synthesis of mammalian cells. I. The two classes of membrane-associated ribosomes. J Mol Biol. 1971 Jul 28;59(2):227–241. doi: 10.1016/0022-2836(71)90048-9. [DOI] [PubMed] [Google Scholar]
- Sheldon R., Jurale C., Kates J. Detection of polyadenylic acid sequences in viral and eukaryotic RNA(polu(U)-cellulose columns-poly(U) filters-fiberglass-HeLa cells-bacteriophage T4). Proc Natl Acad Sci U S A. 1972 Feb;69(2):417–421. doi: 10.1073/pnas.69.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. E., Marcker K. A. N-formylmethionyl transfer RNA in mitochondria from yeast and rat liver. J Mol Biol. 1968 Dec 14;38(2):241–243. doi: 10.1016/0022-2836(68)90409-9. [DOI] [PubMed] [Google Scholar]
- Swanson R. F., Dawid I. B. The mitochondrial ribosome of Xenopus laevis. Proc Natl Acad Sci U S A. 1970 May;66(1):117–124. doi: 10.1073/pnas.66.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesco C., Penman S. The cytoplasmic RNA of HeLa cells: new discrete species associated with mitochondria. Proc Natl Acad Sci U S A. 1969 Jan;62(1):218–225. doi: 10.1073/pnas.62.1.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMSON A. R., SCHWEET R. ROLE OF THE GENETIC MESSAGE IN INITIATION AND RELEASE OF THE POLYPEPTIDE CHAIN. Nature. 1964 May 2;202:435–437. doi: 10.1038/202435a0. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A., Penman S. Processing of 45 s nucleolar RNA. J Mol Biol. 1970 Jan 28;47(2):169–178. doi: 10.1016/0022-2836(70)90337-2. [DOI] [PubMed] [Google Scholar]
- Zylber E., Vesco C., Penman S. Selective inhibition of the synthesis of mitochondria-associated RNA by ethidium bromide. J Mol Biol. 1969 Aug 28;44(1):195–204. doi: 10.1016/0022-2836(69)90414-8. [DOI] [PubMed] [Google Scholar]


